The scientific substantiation of many electrical phenomena became possible thanks to Coulomb’s experiments, on the basis of which the scientist introduced the term “point electric charge.” While exploring the nature of electrification, the French physicist, using the torsion balance he invented, discovered the law of interaction of point charges, known to us as Coulomb's law.
Subsequently, this fundamental law helped scientists form an understanding of the structure of atoms and explain the nature of electricity. This contributed to the creation of sources of electric current, without which the current level of scientific and technological progress would not have been achieved.
Story
Thinkers even before our era paid attention to the existence of electric charges. However, they were not able to explain their nature, much less describe their interaction.
Many centuries passed before scientists began to seriously study electrical phenomena, which led them to discoveries in this area. In particular, William Gilbert, back in the 16th century, not understanding the nature of electricity, called bodies that attracted other substances electrified.
In 1729, observing the electrification of various bodies, Charles Dufay came to the conclusion that there were two types of charges, which he called “glass” (since they manifested themselves on a glass rod) and “resin” (arising from the electrification of resins). Later, Benjamin Franklin replaced the terms "glass" and "resin" with the more general terms "positive" and "negative." We still use these terms today.
Despite the fact that these researchers understood the fact of charge distribution, they were unable to explain the nature of the phenomenon. The physicist C. Coulomb came close to understanding elementary particles as charge carriers. The term “point charge” he coined helped the scientist understand the interaction of elementary particles, which led him to the discovery of the law.
Based on his discovery, the physicist could already explain the reason for the interaction of point charged bodies (see Fig. 1).
Rice. 1. Interaction of electrified bodies
The discreteness (indivisibility) of elementary charged particles was proved by Robert Millikan. The scientist confirmed that a charged body contains an integer number of elementary particles. He came to the conclusion that charge divisibility has a limit. The carrier of the elementary charge is the electron.
Figure 2 shows an experiment confirming charge fissibility. Experience shows that division is multiple, which suggests the existence of elementary particles.
Rice. 2. Charge divisibility
A complete picture emerged after the publication of the visual planetary model of the atom proposed by Rutherford. The model assumes that an atom consists of a nucleus around which electrons revolve. This is a rather simplified model, but it has already explained many electrical processes, including the electrification of bodies.
Rice. 3. Modern interpretation of the planetary model of the atom
What is an electric charge?
This term means that a charged body is capable of creating an electric field. In a broader sense, charge is the amount of electricity - a scalar quantity that is a source of an electromagnetic field and participates in the processes of electromagnetic interactions. Electric charge cannot exist without a carrier.
The elementary carriers of negative charges are electrons. The antipode of an electron is a positron - a stable antiparticle, equal in mass to an electron, but with a “+” sign. There is another stable, positively charged elementary particle - the proton.
Particles charged with fractional parts (quarks) can only exist as part of hadrons, so they are not considered carriers.
The charged protons that make up the nucleus of an atom are tightly bound together by nuclear forces. They cannot escape freely from the nucleus of an atom. Therefore, an ion is considered to be a free carrier of a positive charge - an atom from whose orbit an electron has been removed. The formation of negative ions occurs due to the addition of free electrons to them.
The charge of neutral atoms and molecules is zero, and the number of positive and negative ions in the cells of crystal lattices is compensated. Therefore, bodies under normal conditions are electrostatically neutral. There is no interaction between neutral atoms.
Properties
It has been established that the stationary charge q is inextricably linked with the electric field, a representative of a special type of matter. The field is the material carrier of interaction between elementary particles. This property of the field manifests itself even in the absence of matter between the interacting bodies.
The electric field acts with a force F on a test charge q′ located at any point in the field.
Vector quantity:
characterizes the action of electricity and is called field strength. Lines whose tangents coincide with the tension vector form tension lines. The density of the tension lines determines the magnitude of the tension.
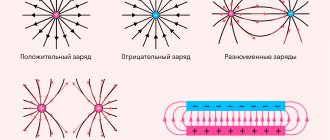
The electrostatic field strength lines of a point charge represent rays leaving one point (for positive) or entering a point (for negative) (see Fig. 4).
Rice. 4. Field strength lines
The electrostatic interaction of electromagnetic fields can be observed in the behavior of charged balls. If an ebonite or glass rod is electrified by friction and brought closer to tiny elderberry balls, then we will see how, as a result of force interactions, the particles repel (if they are of the same sign) or attract (if they are of different signs).
The saturation of free charge carriers of different substances is not the same. Most free electrons are found in metals. Since charged electrons are able to move under the influence of an electric field, they are the main transporters of electric current in metals. In this case, the movement of electrons does not lead to any chemical changes.
Charge transfer in molten salts or acid solutions is carried out by ions. They can be charged either positively or negatively. Unlike metals, the redistribution of charges in these liquids is accompanied by chemical reactions. Therefore, solutions are called conductors of the second kind, that is, those that, under the influence of direct currents, lead to a change in the chemical composition of the substance.
Thus, substances are conventionally divided according to the type of conductivity:
- conductors of the first kind (metals);
- conductors of the second kind (salt, alkaline and acid solutions);
- semiconductors (electron-hole conductivity);
- dielectrics (substances that are unable to conduct electricity due to the lack of free carriers).
General information
Surprisingly, we encounter static electricity every day - when we pet our beloved cat, comb our hair, or pull on a synthetic sweater. So we ourselves inevitably become generators of static electricity. We literally bathe in it, because we live in the strong electrostatic field of the Earth. This field arises due to the fact that it is surrounded by the ionosphere, the upper layer of the atmosphere - an electrically conductive layer. The ionosphere was formed under the influence of cosmic radiation and has its own charge. While doing everyday things like heating food, we don’t think at all about the fact that we are using static electricity when we turn on the gas supply valve on a burner with automatic ignition or bring an electric lighter to it.
Charge interaction
Numerous experiments have shown that charged elementary particles interact with each other. Carriers of like charges repel, and carriers of unlike charges attract (see Fig. 5).
Rice. 5. Interaction of elementary particles
The force of interaction of point charges is determined by the formula resulting from Coulomb’s law: F = (k*q1*q2)/r2 , where q1 and q2 are two charged points located at a distance r, and k is a coefficient, the dimension of which depends on the chosen system measurements, and the value depends on the properties of the environment. Coulomb's law is one of the fundamental laws of physics.
Rice. 6. Interpretation of Coulomb's law
Electrostatic field potential
An electric field with intensity \( \vec{E} \) does work when moving a charge \( q \). The work \( A \) of the electrostatic field is calculated by the formula:
where \( d \) is the distance over which the charge moves, \( \alpha \) is the angle between the vectors of the electric field strength and the charge movement.
Important! This formula is applicable to finding work only in a uniform electrostatic field.
The work of electrostatic field forces when moving a charge from one point in the field to another does not depend on the shape of the trajectory, but is determined only by the initial and final position of the charge.
potential if the work done by forces to move a charge along a closed path is zero.
Important! The work done by the electrostatic field forces when moving a charge along any closed trajectory is zero. The electrostatic field is potential.
The work of the electrostatic field to move a charge is equal to the change in potential energy, taken with the opposite sign. In electrodynamics, energy is usually denoted by the letter \( W \), since the letter \( E \) denotes field strength:
The potential energy of a charge \( q \) placed in an electrostatic field is proportional to the magnitude of this charge. The potential energy of interaction of charges is calculated relative to the zero level (similar to the potential energy of the gravity field). The choice of the zero level of potential energy is determined based on considerations of convenience when solving the problem.
Law of conservation of electric charge
It has been experimentally established that in a closed system one of the fundamental laws of physics is satisfied - the conservation law. In an isolated system, the total charge does not disappear, but persists over time. In addition, it is quantized, that is, it changes in portions that are multiples of the charge of the elementary particle.
The algebraic sum of charges is a constant value: q1 + q2 + … + qn = const (see Fig. 7).
Rice. 7. Preserve static electricity
The law was formulated by B. Franklin (1747) and confirmed by M. Faraday in 1843.
Literature
- Burov L.I., Strelchenya V.M. Physics from A to Z: for students, applicants, tutors. – Mn.: Paradox, 2000. – 560 p.
- Myakishev G.Ya. Physics: Electrodynamics. 10-11 grades: textbook. For in-depth study of physics / G.Ya. Myakishev, A.Z. Sinyakov, B.A. Slobodskov. – M.Zh. Bustard, 2005. – 476 p.
- Physics: Textbook. allowance for 10th grade. school and advanced classes studied physicists/ O. F. Kabardin, V. A. Orlov, E. E. Evenchik and others; Ed. A. A. Pinsky. – 2nd ed. – M.: Education, 1995. – 415 p.
- Elementary physics textbook: Study guide. In 3 volumes / Ed. G.S. Landsberg: T. 2. Electricity and magnetism. – M: FIZMATLIT, 2003. – 480 p.
Measurement methods
The simplest measuring device is an electroscope. It consists of two foil petals located on a metal rod. The structure is covered with a glass cover.
If you touch the rod with an electrified body, the petals become electrified. Since the signs on them are the same, the Coulomb force will push them in different directions. By the magnitude of the deflection angle, you can estimate the amount of static electricity received by the petals.
A more complex device is an electrometer (schematic representation in Fig. 8). The device consists of an electrometer rod, a pointer and a scale. The principle of operation is similar to an electroscope (the arrow is repelled from the rod). Thanks to the presence of a scale, the deflection of the electrometer needle shows the quantitative value of the transferred electricity.
Rice. 8. Schematic representation of an electrometer
We have already mentioned that Coulomb used torsion balances in his experiments. This measuring device allowed the scientist to discover the famous law named after him.