The author of the article is a professional tutor, author of textbooks for preparing for the Unified State Exam Igor Vyacheslavovich Yakovlev
Topics of the Unified State Examination codifier: electrification of bodies, interaction of charges, two types of charge, law of conservation of electric charge.
Electromagnetic interactions are among the most fundamental interactions in nature. The forces of elasticity and friction, gas pressure and much more can be reduced to electromagnetic forces between particles of matter. Electromagnetic interactions themselves are no longer reduced to other, deeper types of interactions.
An equally fundamental type of interaction is gravity - the gravitational attraction of any two bodies. However, there are several important differences between electromagnetic and gravitational interactions.
1. Not just any, but only charged bodies (having an electric charge) can participate in electromagnetic interactions.
2. Gravitational interaction is always the attraction of one body to another. Electromagnetic interactions can be either attractive or repulsive.
3. Electromagnetic interaction is much more intense than gravitational interaction. For example, the force of electrical repulsion between two electrons is several times greater than the force of their gravitational attraction to each other.
Every charged body has a certain amount of electric charge. Electric charge is a physical quantity that determines the strength of electromagnetic interaction between natural objects. The unit of charge is the coulomb (C).
Electric charge
It is impossible to give a brief definition of charge that is satisfactory in all respects. We are accustomed to finding understandable explanations for very complex formations and processes such as the atom, liquid crystals, the distribution of molecules by speed, etc. But the most basic, fundamental concepts, indivisible into simpler ones, devoid, according to science today, of any internal mechanism, can no longer be briefly explained in a satisfactory manner. Especially if objects are not directly perceived by our senses. It is these fundamental concepts that electric charge refers to.
Let's first try to figure out what an electric charge
, and what is hidden behind the statement is
that this body or particle has an electric charge
.
You know that all bodies are built from the smallest particles, indivisible into simpler (as far as science now knows) particles, which are therefore called elementary
. All elementary particles have mass and due to this they are attracted to each other. According to the law of universal gravitation, the force of attraction decreases relatively slowly as the distance between them increases: inversely proportional to the square of the distance. In addition, most elementary particles, although not all, have the ability to interact with each other with a force that also decreases in inverse proportion to the square of the distance, but this force is a huge number of times greater than the force of gravity. Thus, in the hydrogen atom, schematically shown in Figure 1, the electron is attracted to the nucleus (proton) with a force 1039 times greater than the force of gravitational attraction.
Rice. 1
If particles interact with each other with forces that slowly decrease with increasing distance and are many times greater than the forces of gravity, then these particles are said to have an electric charge. The particles themselves are called charged
.
There are particles without an electric charge, but there is no electric charge without a particle
.
Interactions between charged particles are called electromagnetic
. When we say that electrons and protons are electrically charged, this means that they are capable of interactions of a certain type (electromagnetic), and nothing more. The lack of charge on the particles means that it does not detect such interactions. Electric charge determines the intensity of electromagnetic interactions, just as mass determines the intensity of gravitational interactions. Electric charge is the second (after mass) most important characteristic of elementary particles, which determines their behavior in the surrounding world.
Thus
Electric charge
is a physical scalar quantity that characterizes the property of particles or bodies to enter into electromagnetic force interactions.
Electric charge is denoted by the letters q
or
Q.
_
Just as in mechanics the concept of a material point is often used, which makes it possible to significantly simplify the solution of many problems, when studying the interaction of charges, the concept of a point charge is effective. Point charge
- this is a charged body whose dimensions are significantly less than the distance from this body to the observation point and other charged bodies. In particular, if they talk about the interaction of two point charges, they thereby assume that the distance between the two charged bodies under consideration is significantly greater than their linear dimensions.
All atoms are unique
The atoms are very similar, but the different numbers of protons make them a unique type of element. For example, oxygen atoms have 8 protons, hydrogen atoms have only 1, and gold atoms have 79. You can tell a lot about an atom just by counting its protons. These elementary particles are located in the core itself. They were originally thought to be a fundamental particle, but recent research has shown that protons are made up of smaller ingredients called quarks.
Electric charge of an elementary particle
The electric charge of an elementary particle is not a special “mechanism” in the particle that could be removed from it, decomposed into its component parts and reassembled. The presence of an electric charge on an electron and other particles only means the existence of certain interactions between them.
In nature there are particles with charges of opposite signs. The charge of a proton is called positive
, and the electron is
negative
. The positive sign of a charge on a particle does not mean, of course, that it has any special advantages. The introduction of charges of two signs simply expresses the fact that charged particles can both attract and repel. If the charge signs are the same, the particles repel, and if the charge signs are different, they attract.
There is currently no explanation for the reasons for the existence of two types of electric charges. In any case, no fundamental differences are found between positive and negative charges. If the signs of the electric charges of particles changed to the opposite, then the nature of electromagnetic interactions in nature would not change.
Positive and negative charges are very well balanced in the Universe. And if the Universe is finite, then its total electric charge is, in all likelihood, equal to zero.
The most remarkable thing is that the electric charge of all elementary particles is strictly the same in magnitude. There is a minimum charge called elementary
, which all charged elementary particles possess. The charge can be positive, like a proton, or negative, like an electron, but the charge modulus is the same in all cases.
It is impossible to separate part of the charge, for example, from an electron. This is perhaps the most surprising thing. No modern theory can explain why the charges of all particles are the same, and is not able to calculate the value of the minimum electric charge. It is determined experimentally using various experiments.
In the 1960s, after the number of newly discovered elementary particles began to grow alarmingly, it was hypothesized that all strongly interacting particles are composite. More fundamental particles were called quarks. What was striking was that quarks should have a fractional electric charge: 1/3 and 2/3 of the elementary charge. To build protons and neutrons, two types of quarks are enough. And their maximum number, apparently, does not exceed six.
CONTENT
- 1 As one
- 2 Quantization 2.1 Costs less than elementary charge
- 2.2 What is a charge quantum?
- 2.3 No fractional charges
- 3.1 In terms of Avogadro’s constant and Faraday’s constant
Unit of measurement of electric charge
It is impossible to create a macroscopic standard of a unit of electric charge, similar to the standard of length - a meter, due to the inevitable leakage of charge. It would be natural to take the charge of an electron as one (this is now done in atomic physics). But at the time of Coulomb, the existence of electrons in nature was not yet known. In addition, the electron's charge is too small and therefore difficult to use as a standard.
In the International System of Units (SI), the unit of charge is the coulomb
set using the current unit:
1 coulomb (C) is the charge passing through the cross-section of a conductor in 1 s at a current of 1 A.
A charge of 1 C is very large. Two such charges at a distance of 1 km would repel each other with a force slightly less than the force with which the globe attracts a load weighing 1 ton. Therefore, it is impossible to impart a charge of 1 C to a small body (about a few meters in size). Repelling from each other, charged particles would not be able to stay on such a body. No other forces exist in nature that would be capable of compensating for Coulomb repulsion under these conditions. But in a conductor that is generally neutral, it is not difficult to set a charge of 1 C in motion. Indeed, in an ordinary light bulb with a power of 100 W at a voltage of 127 V, a current is established that is slightly less than 1 A. At the same time, in 1 s a charge almost equal to 1 C passes through the cross-section of the conductor.
Determination of the cross-section of wires or cables based on the permissible voltage loss
The selection of the cross-section of conductors in the cable network must be made according to the permissible voltage loss, which is set in such a way that voltage deviations for all electrical equipment connected to this network do not go beyond the permissible limits.
Rated voltages at the output of power supply systems (according to GOST 21128-83):
According to GOST 13109-97:
- The normally permissible value of steady-state voltage deviation is ±5.
- The maximum permissible value of steady-state voltage deviation is ±10.
Line active and inductive reactance
The active resistance of the line (Ohm/km) is equal to:
Value of inductive reactance of conductors Calculation of a network based on voltage loss without taking into account the inductive reactance of wires is permissible in the following cases:
- for DC network;
- AC at cosφ = 1
- for networks made with cables or insulated wires laid in pipes on rollers or insulators, if their cross-section does not exceed the values indicated in the table below.
Formulas for calculating the cross-section of conductors for a given voltage loss
Three-phase AC line:
Two-wire AC or DC line:
Where γ is the conductivity of the wire material, m/(Ohm×mm2);
Un — rated network voltage, kV (for a three-phase network Un — phase-to-phase voltage);
∆Uadd – permissible voltage loss in the line, the cross-section of which is determined, %.
F—conductor cross-section, mm2;
∑P∙L=P1∙L1+P2∙L2+…—the sum of the products of the loads flowing along the sections of the line and the length of these sections; loads should be expressed in kilowatts, lengths in meters;
∑Iа∙L= Iа1 ∙L1+ Iа2 ∙L2+…—the sum of the products of the active components of currents passing through the sections and the lengths of the sections;
Currents must be expressed in amperes, lengths in meters.
The active components of the current (A) are determined by multiplying the current values by the power factor values Ia = I∙ cos ɸ.
Electrometer
An electrometer is used to detect and measure electrical charges.
. The electrometer consists of a metal rod and a pointer that can rotate around a horizontal axis (Fig. 2). The rod with the arrow is fixed in a plexiglass sleeve and placed in a cylindrical metal case, closed with glass covers.
Operating principle of the electrometer
. Let's touch the positively charged rod to the electrometer rod. We will see that the electrometer needle deviates by a certain angle (see Fig. 2). The rotation of the arrow is explained by the fact that when a charged body comes into contact with the electrometer rod, electrical charges are distributed along the arrow and the rod. Repulsive forces acting between like electric charges on the rod and the pointer cause the pointer to rotate. Let's electrify the ebonite rod again and touch the electrometer rod with it again. Experience shows that with increasing electric charge on the rod, the angle of deviation of the arrow from the vertical position increases. Consequently, by the angle of deflection of the electrometer needle, one can judge the value of the electric charge transferred to the electrometer rod.
Rice. 2
Size?
The size of the electron is unknown;
it may turn out to be a point object with no size, or it may have an extremely small size, the radius of which does not exceed 10-18 m. This is at least 100,000,000 times smaller than the radius of an atom. Otherwise, we would see evidence of electron size in experiments. What does an electron actually look like? As I wrote in the article about atoms, defining the concept of the size of an elementary particle is difficult, since an electron, although it is called a particle, is not some speck of dust or grain of salt or sand. It also has wave properties. In an atom, the electrons are, in a sense, distributed throughout the atom, just like a sound wave from a drum. In this sense, being inside an atom, they have the size of the entire atom.
But this is a contextual size, not an inherent size of the electron itself. I will continue to call this “contextual size.” Change the context—take an electron out of an atom, put it in a small metal box—and the electron distribution can grow or shrink. A proton, on the other hand, has an inherent size that is about 100,000 times smaller than an atom. In no sense can you make a proton smaller than its inherent size without breaking it apart. In short, the contextual size cannot be smaller than the internal size. By reducing the contextual size of the electron to a minimum, mainly through the scattering of high-energy electrons from other particles, we looked for their internal size. So far nothing has been found.
So, we can say that experiments show that the inherent size of an electron is less than 10-18 m. And how far an electron travels as a wave depends on the context.
Properties of electric charge
The totality of all known experimental facts allows us to highlight the following properties of the charge:
- There are two types of electric charges, conventionally called positive and negative. Positively
charged bodies are those that act on other charged bodies in the same way as glass electrified by friction with silk.
Bodies that act in the same way as ebonite electrified by friction with wool are called negatively The choice of the name “positive” for charges arising on glass, and “negative” for charges on ebonite, is completely random. - Charges can be transferred (for example, by direct contact) from one body to another. Unlike body mass, electric charge is not an integral characteristic of a given body. The same body under different conditions can have a different charge.
- Like charges repel, unlike charges attract. This also reveals the fundamental difference between electromagnetic forces and gravitational ones. Gravitational forces are always attractive forces.
- An important property of an electric charge is its discreteness
.
This means that there is some smallest, universal, further indivisible elementary charge, so the charge q
of any body is a multiple of this elementary charge: \(~q = N \cdot e\) , where
N
is an integer,
e
is the value of the elementary charge.
According to modern concepts, this charge is numerically equal to the electron charge e
= 1.6∙10-19 C.
Since the value of the elementary charge is very
small, for most of the charged bodies observed and
used
in practice, the number
N
is very large, and the discrete nature of the charge change does not appear. Therefore, it is believed that under normal conditions the electric charge of bodies changes almost continuously. - Law of conservation of electric charge
.
Inside a closed system, for any interactions, the algebraic sum of electric charges remains constant:
\(~q_1 + q_2 + \ldots + q_n = \operatorname{const}\) .
an isolated (or closed) system
a system of bodies into which electric charges are not introduced from the outside and are not removed from it.
Nowhere and never in nature does an electric charge of the same sign appear or disappear. The appearance of a positive electric charge is always accompanied by the appearance of an equal negative charge. Neither positive nor negative charge can disappear separately; they can only mutually neutralize each other if they are equal in modulus.
This is how elementary particles can transform into each other. But always during the birth of charged particles, the appearance of a pair of particles with charges of the opposite sign is observed. The simultaneous birth of several such pairs can also be observed. Charged particles disappear, turning into neutral ones, also only in pairs. All these facts leave no doubt about the strict implementation of the law of conservation of electric charge.
The reason for the conservation of electric charge is still unknown.
Weight!
The electron has mass - it is small compared to the mass of any atom, so it can usually be forgotten in elementary chemistry classes, but it is not so small that it is forgotten in particle physics and even in understanding the structure of atoms. Although electrons do not contribute significantly to the mass of an atom, the mass of an electron is necessary to determine the size of an atom. This, in particular, is the importance of the field and the Higgs particle. This mass can be written in different ways, and each way gives you a different perspective:
- It is equal to approximately 9 × 10-31 kg = 0.000 000 000 000 000 000 000 000 000 000 9 kg.
- It is equal to approximately 0.05% (more precisely, 1/1838) of the mass of a hydrogen atom, the lightest atom in nature. Most of its mass is contained in its core.
- The energy stored in the electron mass, E = mc2, is equal to 0.000 511 GeV. This is 200,000 times the energy carried by one green photon. In particle physics, the mass of a particle is often written in terms of the inverse relationship between energy and mass: for a stationary particle, m = E/c2. In these terms, the mass of the electron is 0.000511 GeV/c2.
Electrification of the body
Macroscopic bodies are, as a rule, electrically neutral. An atom of any substance is neutral because the number of electrons in it is equal to the number of protons in the nucleus. Positively and negatively charged particles are connected to each other by electrical forces and form neutral systems.
A large body is charged when it contains an excess number of elementary particles with the same charge sign. Negative
the charge of a body is due to the excess of electrons compared to protons, and
the positive
charge is due to their deficiency.
In order to obtain an electrically charged macroscopic body or, as they say, to electrify
it, you need to separate part of the negative charge from the positive charge associated with it.
The easiest way to do this is with friction. If you run a comb through your hair, a small part of the most mobile charged particles - electrons - will move from the hair to the comb and charge it negatively, and the hair will become positively charged. When electrified by friction, both bodies acquire charges of opposite sign, but equal in magnitude.
It is very simple to electrify bodies using friction. But explaining how this happens turned out to be a very difficult task.
1 version
. When electrifying bodies, close contact between them is important. Electrical forces hold electrons inside the body. But for different substances these forces are different. During close contact, a small part of the electrons of the substance in which the connection of electrons with the body is relatively weak passes to another body. The electron movements do not exceed the interatomic distances (10-8 cm). But if the bodies are separated, then both of them will be charged. Since the surfaces of bodies are never perfectly smooth, the close contact between bodies necessary for transition is established only on small areas of the surfaces. When bodies rub against each other, the number of areas with close contact increases, and thereby the total number of charged particles passing from one body to another increases. But it is not clear how electrons can move in such non-conducting substances (insulators) as ebonite, plexiglass and others. They are bound in neutral molecules.
2 version
. Using the example of an ionic LiF crystal (insulator), this explanation looks like this. During the formation of a crystal, various types of defects arise, in particular vacancies - unfilled spaces at the nodes of the crystal lattice. If the number of vacancies for positive lithium ions and negative fluorine ions is not the same, then the crystal will be charged in volume upon formation. But the charge as a whole cannot be retained by the crystal for long. There is always a certain amount of ions in the air, and the crystal will pull them out of the air until the charge of the crystal is neutralized by a layer of ions on its surface. Different insulators have different space charges, and therefore the charges of the surface layers of ions are different. During friction, the surface layers of ions are mixed, and when the insulators are separated, each of them becomes charged.
Can two identical insulators, for example the same LiF crystals, be electrified by friction? If they have the same own space charges, then no. But they can also have different own charges if the crystallization conditions were different and a different number of vacancies appeared. As experience has shown, electrification during friction of identical crystals of ruby, amber, etc. can actually occur. However, the above explanation is unlikely to be correct in all cases. If bodies consist, for example, of molecular crystals, then the appearance of vacancies in them should not lead to charging of the body.
Another way to electrify bodies is to expose them to various radiations.
(in particular, ultraviolet, x-ray and
γ
-radiation). This method is most effective for electrifying metals, when, under the influence of radiation, electrons are knocked out from the surface of the metal and the conductor acquires a positive charge.
Electrification through influence
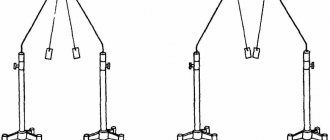
.
The conductor is charged not only upon contact with a charged body, but also when it is at some distance. Let's explore this phenomenon in more detail. Let's hang light sheets of paper on an insulated conductor (Fig. 3). If the conductor is not charged at first, the leaves will be in the non-deflected position. Let us now bring an insulated metal ball, highly charged, to the conductor, for example, using a glass rod. We will see that the sheets suspended at the ends of the body, at points a
and
b
, are deflected, although the charged body does not touch the conductor.
The conductor was charged through influence, which is why the phenomenon itself was called “ electrification through influence
” or “
electrical induction
”.
Charges obtained through electrical induction are called induced
or
induced
.
The leaves suspended at the middle of the body, at points a
' and
b
', do not deviate.
This means that induced charges arise only at the ends of the body, and its middle remains neutral, or uncharged. By bringing an electrified glass rod to the sheets suspended at points a
and
b
, it is easy to verify that the sheets at point
b
repel from it, and the sheets at point
a
are attracted. This means that at the remote end of the conductor a charge of the same sign appears as on the ball, and on nearby parts charges of a different sign arise. By removing the charged ball, we will see that the leaves will go down. The phenomenon proceeds in a completely similar way if we repeat the experiment by charging the ball negatively (for example, using sealing wax).
Rice. 3
From the point of view of electronic theory, these phenomena are easily explained by the existence of free electrons in a conductor. When a positive charge is applied to a conductor, electrons are attracted to it and accumulate at the nearest end of the conductor. A certain number of “excess” electrons appear on it, and this part of the conductor becomes negatively charged. At the far end there is a lack of electrons and, therefore, an excess of positive ions: a positive charge appears here.
When a negatively charged body is brought close to a conductor, electrons accumulate at the far end, and an excess of positive ions is produced at the near end. After removing the charge that causes the movement of electrons, they are again distributed throughout the conductor, so that all parts of it are still uncharged.
The movement of charges along the conductor and their accumulation at its ends will continue until the influence of excess charges formed at the ends of the conductor balances the electrical forces emanating from the ball, under the influence of which the redistribution of electrons occurs. The absence of charge at the middle of the body shows that the forces emanating from the ball and the forces with which the excess charges accumulated at the ends of the conductor act on free electrons are balanced here.
Induced charges can be separated if, in the presence of a charged body, the conductor is divided into parts. Such an experience is depicted in Fig. 4. In this case, the displaced electrons can no longer return back after removing the charged ball; since there is a dielectric (air) between both parts of the conductor. Excess electrons are distributed throughout the left side; lack of electrons at point b
is partially replenished from the region of point
b
', so that each part of the conductor turns out to be charged: the left - with a charge opposite in sign to the charge of the ball, the right - with a charge of the same name as the charge of the ball.
Not only the leaves at points a
and
b
, but also the previously stationary leaves at points
a
' and
b
'.
Rice. 4
Notes
- Or, more precisely, 1.602176487(40)⋅10−19 Cl.
- Or, more precisely, 4.803250(21)⋅10−10 SGSE units.
- The usual instability for a positron associated with the annihilation of an electron-positron pair is not considered.
- But this is far from the only way to electrify bodies. Electrical charges can arise, for example, under the influence of light
- Sivukhin D.V.
General course in physics. - M.: Fizmatlit; Publishing house MIPT, 2004. - T. III. Electricity. - P. 16. - 656 p. — ISBN 5-9221-0227-3. - An electrically closed system is a system in which electrically charged particles cannot penetrate through the surface bounding it (a system that does not exchange charges with external bodies).
Literature
- Burov L.I., Strelchenya V.M. Physics from A to Z: for students, applicants, tutors. – Mn.: Paradox, 2000. – 560 p.
- Myakishev G.Ya. Physics: Electrodynamics. 10-11 grades: textbook. For in-depth study of physics / G.Ya. Myakishev, A.Z. Sinyakov, B.A. Slobodskov. – M.Zh. Bustard, 2005. – 476 p.
- Physics: Textbook. allowance for 10th grade. school and advanced classes studied physicists/ O. F. Kabardin, V. A. Orlov, E. E. Evenchik and others; Ed. A. A. Pinsky. – 2nd ed. – M.: Education, 1995. – 415 p.
- Elementary physics textbook: Study guide. In 3 volumes / Ed. G.S. Landsberg: T. 2. Electricity and magnetism. – M: FIZMATLIT, 2003. – 480 p.