Especially deeply discharged, as in today’s experience in the video.
Especially those that were in a state of partial charge (PSoC) for some time, as a result of which they were sulfated. Considering the inevitable self-discharge during storage and undercharging under the hood, sooner or later this is the fate of almost every battery. Especially worn AGMs that are prone to high heat. Especially, oddly enough, the most reliable and durable batteries in the premium segment, whose dense separators prevent both the destruction of the plates and the mixing of the electrolyte. Especially when there are no plugs to access the electrolyte, as in most modern batteries.
This is because batteries - the batteries of our vehicles, uninterruptible power supplies and renewable energy systems - have specific characteristics of the current-voltage characteristic (volt-ampere characteristics), determined by physical and chemical properties.
This will be discussed using the example of a deeply discharged hybrid (Sb/Ca) Tyumen Standard 6ST-60L.
Some useful links:
- A striking example of the consequences of self-discharge when storing a new battery is discussed in detail in the first part of a large test of 6 domestic batteries.
- The oxygen recombination cycle, which causes “thermal runaway” of worn-out AGMs, is described in the article about the first domestic AGM.
- A method for determining the individual voltage for completing the charge of a specific battery using an adaptive charger in the absence of access to the electrolyte is given in the first part of a large test of 6 batteries of foreign brands.
- How progressive undercharging kills batteries, and whether they can be restored after this, as well as the phenomenon of imaginary, or surface, charge is described here.
- And here you can read about the “secret”, “high-voltage” charging stage, including for AGM, known to professionals and indicated in the instructions from battery manufacturers either explicitly or implicitly.
The laboratory received a Tyumen Standard 6ST-60L battery.
12 V 60 A*h, rated cold cranking current (CCC) 520 A in the EN standard. The battery was used for a year and a half.
The electrolyte level is so low that it does not cover the plates. White crystals of lead sulfate are visible. The car was idle for 2 months due to a gearbox failure. For a Ca+ hybrid battery, unlike Ca/Ca, this is a considerable period in itself. In addition to self-discharge, there was a quiescent current of the security alarm of about 30 mA. Over 2 months, the discharge with this current is 43 A*h. This is almost the entire capacity of a used battery.
The battery is warming up. The open circuit voltage (OCV) is 10.53 V. In the cold 2 hours ago it was 8 V. Let's leave it to warm up at the heat gun for another 2 hours.
Before charging a lead-acid battery of the “wet” (WET) type, that is, with freely splashing electrolyte, you need to make sure that the electrolyte covers the plates.
Otherwise, add distilled water (not tap water, not drinking water, not electrolyte!) to the edges of the plates. (Not to the normal level!) The electrolyte level will rise during the charging process. If you add too much, the electrolyte may spill out of the top of the cans while charging, creating unnecessary problems.
The battery warmed up, the missing water was added. We will charge using the domestic programmable charger Kulon-912.
▍ Current-voltage characteristic
If we use a charger with a classic CC/CV charging mode based on a stabilized power source, we simply need to remember one important point, which becomes a stumbling block every day.
Questions of the same kind are constantly asked about stabilizing current and voltage when charging a battery or powering a particular consumer, as similar as drops of water. “Why do I install 15 volts 3 amps, but the current turns out to be less than 3 amps? The charger produces 3 amperes only at 17 volts, is it defective? “Why am I setting 15.5 volts 6 amps, but the voltage is only 14 volts?”
The fact is that a real consumer of electrical energy, for example, a battery when charging, has its own current-voltage characteristic, in the simplest case described by electrical resistance.
Let's say we have a regulated 100+ W power supply set to 10 volts 10 amps.
If you connect a 1 Ohm resistor to its output, the current at a voltage of 10 V will be exactly 10 A, and according to the Joule-Lenz law, a power of 100 W will be released. This situation is called resistance matching, when both current, voltage, and power are maximum. If the resistor is 10 ohms, the current will be only 1 A, the power will be 10 W. The power source will have active voltage feedback (FE), but the current feedback will not trigger. This is not a malfunction of the power supply, but the logic of its operation and the nature of the resistor.
With a resistance of 10 milliohms and a current of 10 amperes, for example, on a current-measuring shunt, the voltage will be only 0.1 volt, the heat dissipation will be 1 W. Here the current feedback works, but the voltage feedback does not work.
An ideal resistor is the simplest case; it has a linear current-voltage characteristic (VC), and it is constant over time and does not depend on temperature. But if you take the filament of a light bulb, then at the moment of switching on the cold filament has low resistance, a current flows above the working one, the so-called starting current. Let it be 10 amperes, the maximum that the power supply unit (PSU) will produce, at 8 volts. Next, the thread will heat up, its resistance will increase, the current will drop, for example, to 7 A, and the voltage will increase to the specified 10 volts.
This is not a malfunction of the light bulb or power supply, but the physics of their operation. It turns out that an incandescent lamp has a current-voltage characteristic over time, determined by the temperature coefficient of resistance (TCR) of the metal (alloy) of its filament.
By the way, it is for this reason that light bulbs often burn out precisely at the moment they are turned on, when the filament is cold and has low resistance. To prevent an arc discharge from being maintained when the spiral burns out, which can cause an electrical overload, an explosion of the bulb and a fire, inside many light bulbs there is a fuse in the form of a section of thinner wire coming from the base inside the bulb. In a burnt-out light bulb, we often see balls of molten metal stuck to the glass from the inside in the area where this section passed.
To start an electric motor, especially one loaded with some mechanism on the shaft (for example, a refrigerator compressor), greater current and power are needed than to maintain its rotation even when the already running mechanism takes torque and energy from the shaft.
Moreover, the motor windings are not designed for long-term operation in starting mode. Therefore, for many decades, starting capacitors of higher ratings than working ones and thermal starting protection relays have been used, which prevent not only prolonged operation at high current (for example, when a mechanism is jammed), but also several starts in a row for a short time (during power outages ).
So, in technology it is necessary to take into account the current-voltage characteristic of a real consumer and its dynamics over time.
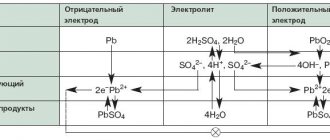
A lead-acid electrochemical cell behaves when charging even more complexly than a light bulb and an electric motor. In addition to the thermodynamic emf (electromotive force), and the voltage drop across the internal resistance (both the emf and the internal resistance depend on the charge level and temperature, both change during charging), polarization appears in a lead battery.
The distribution of ions (that is, charge carriers) in the volume of the battery jar (cell) (where the electric field operates) creates an emf that is added to the voltage at the terminals during charging and subtracted during discharge. This phenomenon can be called a “parasitic supercapacitor”
or
“supercapacitor”.
The dense structure of the separators of modern batteries, especially premium versions (SSB - batteries for start-stop systems, EFB - improved flooded batteries), prevents the drift of ions in the electrolyte and thereby creates the effect of “parasitic electret”,- persistent overvoltage that lasts for a long time.
Also, additional EMF is created by gases - hydrogen and oxygen - in the pores of the active masses. This is already a “parasitic fuel cell”.
The parasitic “supercapacitor” and “fuel cell” in an acid battery have a fairly significant electrical capacity, the charge of which is extended over time.
Therefore, when charging a battery, the voltage at its terminals increases not only by the sum of the thermodynamic EMF of the banks and the voltage drop across the internal resistance, but also as the parasitic capacitances charge. That is, when a charging current of 5% capacity, (3 amperes for 60 A*h) is supplied to a discharged battery with NRC, (a term not identical to EMF for the reasons described above), 12 volts, it will create an overvoltage of only 100-200 millivolts, or even lower.
The same current, supplied to the terminals of a charged battery with an NRC of 12.9 volts, which is only 900 millivolts higher than the discharged one, will soon create an overvoltage, for example, up to 16.7 V, that is, 3.8 volts, which is 25 times higher than the case from the previous paragraph.
Therefore, a charger configured for 15 volts 6 amperes will supply 6A 12.3 V in the first case, in the second the voltage will quickly jump to 15V, and the current will drop to 1 A and even lower. This is not a malfunction of the charger or battery, but the physics and chemistry of a lead battery, and the operation of feedback connections of a stabilized power source.
It can be difficult to predict the correct voltages, currents and times for each charging stage for a given state of a particular battery. In some cases, manufacturers limit themselves to general recommendations, in others they prescribe complex multi-stage charge profiles, such as this one from Tianneng.
Different chargers provide varying degrees of process automation and monitoring and control capabilities. Also, when servicing lead batteries, devices such as load forks, express testers, discharge loads, and means for determining the density of the electrolyte are used - hydrometers and refractometers. The latter are irrelevant if there is no access to plugs for popular MF (maintenance free) batteries.
The word “maintenance-free” does not mean that these batteries do not require periodic stationary charging, and refers only to the electrolyte charged for its entire service life.
Target of stationary charge
- convert all sulfates in the lubricants of the battery plates into charged active masses (AM), - sponge lead is negative and lead oxide is positive, and mix the electrolyte until the acid concentration is uniform, i.e. density of the solution throughout the entire volume of the jars.
This restores operational characteristics, including the ability to quickly and effectively replenish charge
from the vehicle generator after starting the engine, the standard charger after a trip on an electric motorcycle, or the charge controller of the uninterruptible power supply after the external power supply is restored.
Desulfation
is called the process of electrolytic dissociation of old, sparingly soluble sulfates. This is a necessary part of a complete equalizing stationary charge, which restores capacity, current output, and extends the service life of the battery.
Battery
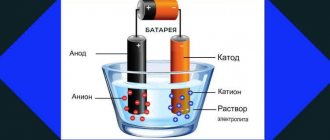
To understand the principle of operation of a battery, a short historical excursion into the appearance of chemical batteries is necessary. The first prototype of the battery dates back to 1800. It was created by Allesandro Volta. By immersing two plates of different metals in acid (zinc and copper), he obtained a direct current across them. During the experiment, the zinc plate gradually dissolved, and the copper plate became covered with gas bubbles. The name of such a battery is “Volt Pillar”.
Energy storage devices work in a similar way, only the chemical reactions are partially reversible; the electric current sent back to the movement of the discharge current restores the chemical composition of the metals of the plates.
The basis of any charge-storing device is two plates of chemically different metals connected by a layer of electrolyte. Such a layer can be liquid (for example, lead versions), or it can be a porous mass impregnated with an electrically conductive composition. The material used to make the battery's electrode plates determines the characteristics of the battery. For lead storage devices used for vehicles, one plate is made of lead, the second consists of its dioxide. Such devices are marked with Pb symbols on the housing. For the most common lithium-ion power supplies among household appliances (Li-Ion), one aluminum electrode is used and the other is copper.
Several such assemblies of plates electrically connected to each other are called a battery. Assembly is necessary to obtain the required voltage. The characteristics of rechargeable batteries directly depend on what characteristics of the battery are present on each pair of metal plates.
All common types of energy storage devices:
The table is not completely complete, as new types of batteries are constantly being developed.
▍ Drip precharge with pulsating current
Let's start restoring our battery. Pendant-912 is equipped with a pulse precharge function. The expediency of this stage is due to the fact that deeply discharged, i.e. An unbalanced battery, when supplied with a standard current of 10% of the capacity, can become very hot, since different sections of the plates will receive different current densities, and different banks will receive different overvoltages.
To avoid this, we set the current to 5% of the rated capacity, for 60 A*h this is 3 A. We make the pulse and pause durations equal, 5 seconds each. Completion of the stage upon reaching the voltage in the pause, i.e. NRC 12 volt.
Why is this necessary?
The battery is discharged for 2 main reasons.
- Determination of capacity. First, the battery is charged 100%, after which time-controlled discharging begins. As a result, you can see the real state of the device. This gives reason to think about whether it is time to change the battery or not.
- Training cycle. This is a charge-discharge cycle. It is often carried out by car owners. And they do this before frost, every year. Such training allows you to increase the resource and extend the service life of the power supply on the machine.
Based on specific circumstances, the car owner will decide whether he needs to discharge his battery or whether there is no need for it.
▍ Main charge stage
The main charge settings are standard for a hybrid battery. The maximum voltage is 14.6 V, the current decrease begins at 14.5 V, the current is 6A, this is 10% of the capacity. But let’s also include asymmetry (reverse): the discharge current is 10% of the charging current, i.e. 0.6 A, charging pulse duration 5 seconds, discharge pulse duration 50% of the charging pulse.
Discharge pulses with an asymmetric (reversible) charge partially remove polarization, thereby increasing the efficiency of charging and desulfation. Some adaptive memory devices, in contrast to classical ones, including programmable ones, use a discharge pulse to analyze the response of the electrochemical system. Discharge pulses, like charging ones, can be modulated, i.e. be packets of shorter pulses and pauses, which allows you to study the internal resistance of the battery at a different frequency.
The end of the stage after 6 hours at the set voltage achieved. It is difficult to predict what the current will be at the end of the main charge. Therefore, it is good that the memory provides such an automation option. We will not activate the recharging and storage stages yet. First, let’s check what the precharge and main charge will lead to with these settings.
The charge lasted 19 hours 34 minutes, the battery received 57.53 Ah. This number gives hope that the battery has not experienced a significant loss of capacity after a deep discharge.
The density of the electrolyte in the banks is from 1.23 to 1.25, which is clearly not enough. There is electrolyte separation and recharging is required.
The tester shows TCP 501 out of 520 A, battery health (SoH, state of health) 96%. These are good indicators, the battery will still serve, but we must take into account that an undercharged battery has a slightly lower internal resistance than a 100% charged one. Now it is 6.20 milliohms.
Difficulty of choice
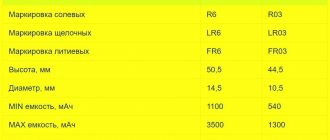
Choosing a battery is not an easy task. At first glance it may seem that this is a very simple device. But in reality it turns out that it is full of characteristics that are important. But don't despair. You basically need to decide on the battery size, type, capacity and starting current. In principle, you can find out all these parameters by inspecting your old battery. Capacitance and inrush current are usually written in large letters in a visible place. For example, 55 Ah 450 Ampere. This is who they are. Above is a table with capacity depending on the vehicle. It can be used to further orientate oneself. The article also provided the sizes of domestic, European and Asian batteries, by which you can understand what type your battery is. Mainly only these batteries are sold on the Russian market.
▍ Recharge phase
We will recharge with a current of 2.2A, this is slightly higher than 1/30 of the capacity, without voltage limitation, until the voltage stops increasing within 2 hours. Unfortunately, the Kulon-912 does not have such a ZDV automation option (zero delta voltage, zero voltage increment), but it does have remote monitoring and control, as well as log recording. Therefore, we will observe the process and complete it manually.
In 21 minutes, the voltage increased by 40 millivolts and amounted to 14.94 volts. We continue monitoring.
At the 49th minute of charging, the voltage dropped to 14.92-14.93 V. We mark 2 hours and turn off the charge.
Almost two hours passed, the voltage dropped to 14.84 V. This is due to a decrease in the internal resistance of the battery, in particular due to its heating. The battery is slightly warm. A total of 5.92 Ah were supplied.
More than a day has passed, NRC is 12.92 V. The density of the electrolyte in the banks is 1.25 - 1.29. Lower density in those jars where water was not added.
Terminal type
European, standard
All domestic and many foreign batteries have cone-type terminals. Thus, the “plus” in European products has a diameter of 19.5 mm, and the “minus” - 17.9 mm.
Asian, thin (JIS)
They differ in size from European ones. Japanese battery terminals can be one of three types:
- T1 – the diameter of the positive terminal is 14.7 mm, and the negative terminal is 13 mm. Their shape is a truncated cone. The narrowest, characteristically “Japanese” terminals, which are most widespread on cars manufactured in Japan.
- T2 - these terminals can be found on high-capacity batteries. Their dimensions are 19.5 and 17.9 mm for plus and minus, respectively.
- T3 – terminals of the third type are flat pins with special holes.
Lateral
The leads for the terminals are located on the side surface of the top cover and have an “internal” thread. Standard for US made vehicles.
Screw
In the form of screws protruding from the top of the battery. They are also the type of terminals used on American batteries.
Bolt-on
For trucks.
▍ Check digit and total
To evaluate the residual capacity, we discharge up to 12 V under a load of 2 amperes. This will be approximately 50% of the capacity.
The discharge was completed, the capacity was 19.48 Ah, as expected. We put it on charge by repeating the 3 steps described above.
After charging and standstill, the NRC is 13.03 V, internal resistance 5.78 mOhm, TCP 537 out of 520 A according to EN. SOH 100%. Great result! The battery has been fully restored. Now let’s measure and, if necessary, adjust the density of the electrolyte.
10-15 cubic centimeters of distilled water added to a jar of a 12-volt battery with an L2 housing will reduce the density of the electrolyte by 0.01. Electrolyte, not water. should only be added if there has been a loss of acid due to electrolyte leakage.
The density in all jars was 1.27-1.28, no correction required.
Battery restoration has been completed and is being returned to the owner. Video version:
The article was written in collaboration with the author of the experiments and videos - Battery Engineer Victor VECTOR.
Safety
In addition to the characteristics of a car battery, knowledge of safety precautions when operating and maintaining the battery is equally important. First of all, pay attention to the following points:
- The battery must be securely secured in the engine compartment of the vehicle;
- When measuring electrolyte density and replacing it, it is necessary to use personal protective equipment (goggles, rubber gloves). Keep baking soda and water nearby in case the electrolyte gets on your skin;
- Do not short-circuit the battery terminals;
- Before charging the battery, remove the caps from the cans of the battery being serviced.
This is what can happen if you don't follow safety rules.
Discharging without removing the battery
To specifically reduce the battery charge, it is not necessary to remove the device from the car. It can be left connected under the hood of the car.
Some cars have a voltmeter on the dashboard. If it's not there, don't worry. Simply connect the device to the battery, observing the polarity.
A simple but effective and relatively fast method. To do this you need:
- make sure there is a voltmeter at the dashboard, or prepare a separate measuring device;
- turn off the engine;
- connect a voltmeter;
- turn on energy consumers powered by a battery;
- use a measuring device to monitor the drop in indicators;
- When the level reaches below 10.9 V, stop the discharge.
The more consumers work simultaneously, the faster the required degree of discharge will be achieved. Therefore, you can turn on the headlights, glass and seat heaters, the heater in the cabin, etc.