It is difficult to overestimate the practical importance of electrolysis - a complex mechanism of physical and chemical processes. Its mechanism is quite simple, but it allows electrolysis to be effectively used in various areas of economic activity - from the metallurgical industry to arts and crafts; it can even be used at home. Electrolysis is studied by physics and chemistry; in each field of science, specific technologies have been developed, which are based on the same principle.
What is electrolysis
To understand what electrolysis is, you need to imagine a system consisting of electrodes with opposite polarities immersed in a liquid electrolyte. Electrolysis is a system of processes that operate through the interaction of system elements in the presence of a direct electric current from an external source and leading to the emergence of an ionic current.
In a simplified form, the electrolysis circuit looks like this.
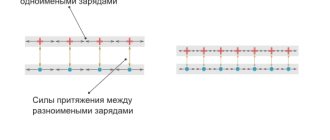
The positive electrode (anode) and negative electrode (cathode) attract electrons of opposite polarity in the liquid medium.
Particles with a negative charge (they are usually called anions) move towards the anode, cations carrying a positive potential move towards the cathode.
At the anode, the anions give up free electrons, producing an oxidation reaction. Cations, on the contrary, take up electrons at the cathode, carrying out a reduction reaction that has the form Men+ + ne → Me (where n is the valency of the metal). This is how the redox process occurs in the system.
Most often, hydrogen and metal ions act as cations, and chlorine or oxygen act as anions.
Table of changes of substances using electrolysis
Strengthening the restorative abilities of substances:
| Na+ | Mg2+ | Al3+ | Zn2+ | Fe3+ | Ni2+ | Sn2+ | Pb2+ | H+ | Cu2 | Ag+ |
| Sodium | Magnesium | Aluminum | Zinc | Iron | Nickel | Tin | Lead | Hydrogen | Copper | Silver |
Strengthening the oxidative abilities of substances:
| I- | Br- | Cl- | OH- | NO3- | CO32- | SO42-. |
| Iodide (salts formed by hydroiodic acid) | Bromide (salts formed by hydrobromic acid) | Chloride (salts formed by hydrochloric acid) | Hydroxide | Nitrate (salts formed by nitric acid) | Carbonate (salts formed by carbonic acid) | Sulfate (salts formed by sulfuric acid) |
| Cathode (negative) | Anode (positive) |
| Reduction of cations after hydrogen | Oxidation of oxygen-free acid anions |
| Reduction of cations with medium activity | Oxidation of oxoacid anions |
| Reduction of the most active cations | Oxidation of hydroxide anions |
| Reduction of hydrogen cations |
Electrolysis and Faraday's laws
Michael Faraday is an English experimental physicist who made several important discoveries concerning electromagnetic phenomena. Electrochemical studies of the nature of reactions, published by the scientist in 1836, allowed him to formulate the laws of electrolysis. They formulate a relationship between the amount of substance produced in the process of an electrochemical reaction and the amount of electricity affecting the electrolyte.
First Law
In general terms, Faraday's first rule of electrolysis sounds like this: the mass of a substance added to the electrode during the reaction is directly proportional to the volume of electricity passed through the electrolyte using the electrodes. Reaction formula:
m = kq = k*I*t
(variable values: q – charge, k – electrochemical equivalent (coefficient) of the substance, I – current strength affecting the electrolyte, t – time of passage of electricity).
Second Law
The following Faraday rule of electrolysis is formulated: the mass of a substance that the electrode will receive when exposed to a certain amount of current is directly proportional to the equivalent mass of the same substance.
This term denotes the molar mass divided by an integer determined by the chemical reaction in which the element participates. In another formulation, the law looks like this: an equal amount of electricity leads to the release of equivalent masses of different elements on the electrodes during electrolysis.
To release one mole of a substance, a certain amount of electricity must be expended, equal to 96,485 C/mol.
This constant became known as the Faraday number. The simplest formulation of the law states: the electrochemical equivalent of each element is directly proportional to its molar mass and inversely proportional to the valence of the same element. Formula:
m = Q/F*A/z
(variable values: m – the desired mass of the resulting substance, Q – the amount of total charge passed through the electrodes, F – Faraday number, A – molar mass, z – chemical valence of the element). By combining all the values described in both laws, we can derive a general formula that determines the mass of the substance collected on the electrodes: m = A*I*t/(n*F) (n is the charge of the ion or the number of electrons participating in the electrolysis reaction).
Story
A device invented by Johann Wilhelm Ritter to develop the electrolysis of water.
Jan Rudolf Deyman and Adrian Paets van Troostwijk used an electrostatic machine in 1789 to produce electricity, which was discharged onto gold electrodes in a Leyden jar filled with water. In 1800, Alessandro Volta invented the voltaic battery, and a few weeks later, English scientists William Nicholson and Anthony Carlyle used it to electrolyze water. In 1806, Humphry Davy reported the results of extensive experiments on the electrolysis of distilled water, concluding that nitric acid was formed at the anode from dissolved atmospheric nitrogen. He used a high-voltage battery and non-reactive electrodes and vessels, such as gold electrode cones, which doubled as vessels covered with wet asbestos. When Zenob Gramm invented the Gramm machine in 1869, electrolysis of water became a cheap method for producing hydrogen. The method of industrial synthesis of hydrogen and oxygen by electrolysis was developed by Dmitry Lachinov in 1888.
Factors affecting electrolysis
Formulas describe the passage of a reaction in an ideal environment, without taking into account many accompanying factors that can change the expected result. In addition to the complex of components taken into account in the laws, the total components of the reaction are influenced by:
- Electrolyte composition. The course of the reaction and its result are affected by foreign impurities that have entered the electrolyte. They are divided into cationic, anionic and organic. Foreign molecules have a more or less negative potential than the main compound, and this greatly interferes with the process. The concentration of organic contaminants (this can be surfactants or oils) has a final permissible value.
- Electricity density. Faraday's laws state that the more powerful the current, the greater the amount of substance that will settle on the electrodes. In practice, an increase in current often causes unfavorable phenomena - intense heating of the electrolyte, concentrated polarization of the electrodes, excessive current voltage. To obtain the expected result from electrolysis, the energy density values that are optimal for each situation must be observed.
- Electrolyte temperature. Its action is ambiguous. On the one hand, as it increases, the intensity of the reaction increases, on the other hand, the activity of foreign impurities increases. Therefore, it is necessary to ensure that the temperature of the liquid is within the optimal range for a particular case, usually 38-45 degrees.
- Electrolyte acid-base balance. The optimal pH value of the medium depends on the specific substance. It is possible to control the speed of electrolysis and its result, bringing it to optimal, if the influence of existing factors is correctly combined. For each type of reaction, the necessary operating modes have been developed experimentally and must be adhered to.
Self-test questions
1. Choose the correct continuation of the phrase “the cathode is...”:
- A positively charged electrode to which positively charged ions are attracted.
- A positively charged electrode to which negatively charged ions are attracted.
- A negatively charged electrode to which positively charged ions are attracted.
- A negatively charged electrode to which negatively charged ions are attracted.
2. Continue the phrase “electrolysis is...”:
- OVR using current.
- Reaction without changing oxidation states using current.
- ORR using catalysts.
- Exchange reaction.
3. How is the anion charged?
- Positively.
- Negative.
- Neutral.
- Has no charge.
4. How does electrolysis of a solution differ from electrolysis of a melt?
- Nothing.
- The solid melts in the melt.
- The presence of water molecules and its dissociation products.
5. If a metal is in the activity series of metals between aluminum and hydrogen, what will be released at the cathode?
- This metal.
- Hydrogen.
- Metal and hydrogen.
- Metal oxide.
During the electrolysis of an aqueous solution of lithium fluoride, what will be released at the anode?
- Fluorine.
- Hydrogen.
- Oxygen.
- Water.
Features of the processes occurring at the cathode and anode
Electrodes are rods made of materials with high electrical conductivity. They can be divided into two categories - active electrodes, which, when oxidized, participate in the exchange of ions, and inert electrodes - made of graphite, coal or platinum, which perform only the function of conductors.
Active electrodes - anodes - can oxidize until completely dissolved in the electrolyte, releasing ions to the cathode.
In order to correctly calculate formulas and obtain the expected result of electrolysis - whether as part of school preparation for the Unified State Exam, exams or during the operation of an enterprise - it is necessary to understand exactly how electrodes of different polarities participate in the course of the reaction. An electrode that has a negative charge, the cathode, attracts ions with the opposite charge “+”, for example, metals: sodium (Na+), potassium (K+), copper (Cu2+), iron (Fe3+), silver (Ag+) and others.
The process of electrolysis at the cathode depends greatly on how active the substance is.
Its position in the series of electrochemical activity of metals will help determine the factor:
- A highly active metal (lithium, sodium, potassium), interacting with the cathode, instead reduces water molecules, from which, in turn, hydrogen is released.
- The average activity of the metal (chromium, iron, cadmium) promotes the formation of both water and substance molecules at the cathode.
- The low activity of the metal (copper, silver) makes it possible to obtain molecules of the substance in its pure form.
Aluminum is considered an intermediate link in the voltage series between high- and medium-active metals.
All metals in the series up to and including it are not reduced at the cathode, releasing only hydrogen from water molecules.
The pure substance helps to obtain the electrolysis of AlCl3 (aluminum chloride) and other highly active metals in an anhydrous melt - the initial mixture breaks down into its component parts, for example, AlCl3 → Al + Cl2.
Anions with “+” tend to the positively charged anode, and cations with “-“ tend to the negatively charged cathode.
They attract electrons from the conductor, oxidizing it, change the charge to positive and are attracted to the cathode.
The anodic process sometimes leads to complete dissolution of the active substance electrode in the electrolyte.
Anions of different elements also differ in their degree of activity. A process such as oxidation has features that depend on additional components of anions:
- Molecules containing oxygen - sulfates, phosphoric acids - during electrolysis oxidize water molecules, releasing oxygen from them.
- Oxygen-free anions, interacting with the anode, release certain halogens: as examples, sulfur is released from sulfides, and chlorine from chlorides. All nonmetals are more easily oxidized than oxygen. The exception is fluorine, which, as the most electronegative substance, as a result of the reaction oxidizes water molecules in solution to oxygen.
- An organic element is oxidized at the anode as follows: the radical attached to the carboxyl group doubles, and the group itself takes the form of a gas (CO2).
Equations
Diagram showing a general chemical equation.
In pure water, a reduction reaction occurs at a negatively charged cathode, in which electrons (e – ) from the cathode are transferred to hydrogen cations to form hydrogen gas. The half-reaction, balanced with acid, is:
Reduction at the cathode: 2 H + (
aq
) + 2e – → H 2 (
g
)
At the positively charged anode, an oxidation reaction occurs, generating oxygen gas and donating electrons to the anode to complete the circuit:
Oxidation at the anode: 2 H 2 O (
l
) → O 2 (
g
) + 4 H + (
aq
) + 4e –
The same half-reactions can also be balanced with the base given below. Not all half-reactions need to be balanced with an acid or base. Many of them, such as water oxidation or reduction, are listed here. To add half a reaction, both must be balanced by an acid or a base. Acid-balanced reactions predominate in acidic (low pH) solutions, while base-balanced reactions predominate in basic (high pH) solutions.
| Cathode (recovery): | 2 H 2 O ( l ) + 2e – | → | H 2 ( g ) + 2 OH – ( aq ) |
| Anode (oxidation): | 2 OH – ( aq. ) | → | 1/2 O 2 ( g ) + H 2 O ( l ) + 2 e – |
Combining any pair of reaction halves results in the same overall decomposition of water into oxygen and hydrogen:
General reaction: 2 H 2 O (
l
) → 2 H 2 (
g
) + O 2 (
g
).
Thus, the number of hydrogen molecules formed is twice the number of oxygen molecules. Assuming that the temperature and pressure are the same for both gases, the hydrogen gas produced therefore has twice the volume of the oxygen gas produced. The number of electrons pushed through water is twice the number of hydrogen molecules generated and four times the number of oxygen molecules generated.
Electrolysis in media
The difference in the principles of electrolysis of melts and solutions is that in the solution, in addition to particles of the substance, there are water molecules that prevent the reduction of some substances at the cathode.
Water
When involved in the electrolysis reaction, H2O water competes with ions of the electrolyte and anode for reduction at the cathode.
Depending on the properties of the substance, during the electrolysis of an aqueous solution, oxygen, cations from the liquid medium, or molecules of the soluble anode can be reduced on the negative electrode.
For example, in the case of NaF electrolysis, the process occurs only for water ions, which are separated into oxygen and hydrogen.
2H2O → 2H2 + O2
Molten salts
Due to the lack of “competition” of water molecules, electrolysis of melts is predicted and calculated more easily than the reaction of a solution.
All metals, regardless of activity, react in the same way.
As an example, consider the electrolysis of molten sodium chloride. When an electric current passes through the NaCl melt, reduction of sodium particles will occur at the cation, and oxidation of the chloride residue at the anode. The general equation in this case looks like this: 2Na+Cl– → 2Na0 + Cl20. In a similar way, the separation of substances occurs during the electrolysis of salts of other metals.
SODIUM HYDROXIDE, HYDROGEN AND CHLORINE ARE PRODUCED BY ELECTROLYSIS OF AN AQUEOUS SOLUTION OF NaCI
Salt solutions
It is more difficult to calculate the result of a solution electrolysis reaction, even if you do not take into account the presence of possible impurities. Indeed, in addition to the molecules of metal salts, when the electrolyte flows, the process is influenced by water molecules, which have a significant impact on the course of the process.
During the electrolysis of salt solutions, either hydrogen, electrolyte cations, or metal molecules will be reduced on the negative electrode.
The result depends on how much external energy is needed in each case - the reaction with the least electricity consumption will be given priority.
Electrolysis of solutions will cause ions with the highest energy potential to rush to the cathode, and the anode will attract anions with the lowest potential.
In the example of electrolysis of a sodium chloride solution at the cathode, only hydrogen can be obtained.
2H+2O +2ē → H20 + 2OH–
Electrolysis of a solution of a salt of carboxylic acids promotes the oxidation of the carbon atom and the release of carbon dioxide occurs
Copper sulfate solution on the anode of a water molecule
2H2O-2 – 4ē → O2 + 4H+
Features of electrolysis in solutions
The result of aqueous electrolysis reactions in the presence of water depends only on the position of the metal in the electrochemical voltage series. The rules of the electrochemical process of solutions at the cathode are as follows:
- Water appears on the negative electrode and oxygen is released if the metal contained in the electrolyte is located to the left of aluminum (included in the list from lithium to aluminum), for example, during electrolysis of a sodium chloride solution.
- The joint reduction of water and metal ions occurs when the metal is in the series from aluminum to hydrogen (from magnesium to lead inclusive).
- Reduction of only the metal at the cathode is possible if the electrolyte substance is located to the right of hydrogen (the series from copper to gold inclusive). Electrolysis of the solution at the anode depends on the composition of the electrode. The active anode is oxidized in any case, and sometimes completely dissolves itself. An inert electrode consisting of graphite, platinum or gold exhibits the following properties:
- In the presence of salts of oxygen-free acids, excluding fluorides, the anode will oxidize.
- Electrolysis of hydroxy acids (such as nitric acid solution HNO3) and fluorine compounds leads to oxidation of water, metal ions will remain in the liquid.
- Passing electricity through an alkali solution will release hydroxide ions from the electrolyte. To achieve a reaction from some acids, such as electrolysis of acetic acid, it is necessary to make a sufficiently dilute aqueous solution so that it begins to show signs of electrical conductivity.
Electrolyte solutions with inert electrodes
To better understand the processes taking place with the participation of inert electrodes, it is worth considering several illustrative examples:
- Sodium hydroxide (NaOH). The position of sodium is to the left of aluminum, therefore, its cathodes will not be reduced from the electrolyte. During cathodic electrolysis of a sodium hydroxide solution, hydrogen is reduced, and 2OH hydroxides at the anode are oxidized to oxygen and water.
- Copper sulfate CuSO4. The place of copper is in the voltage series to the right of hydrogen, so it will be completely reduced at the cathode. The anode will oxidize the water molecules, and the acidic residues will remain in the electrolyte with oxygen.
- Sulfuric acid H2SO4. At the cathode, only hydrogen cations will be reduced; at the anode, the process of water oxidation will take place.
- Sodium sulfate Na2SO4. As in the previous example, sodium cations will remain in the electrolyte, and hydrogen will be reduced at the cathode. The sulfate anion, as an oxygen-containing ion, will also not be able to be released from the liquid.
In gases
Gas can act as an electrolyte only in the presence of an ionizer.
Electricity can only produce the expected reaction at the electrodes by passing through an ionized environment.
In this case, Faraday's laws do not apply. The gas electrolysis process obeys the following rules:
- Only acids that are in a gaseous state and do not contain oxygen, or some gases, are accessible to exposure.
- The reaction will not take place without artificial ionization of the medium, regardless of the strength or voltage of the electricity.
If an electrolyte breaks down into ions, this is electrolytic dissociation.
Laboratory work No. 4.
Electrolysis of an aqueous solution of potassium iodide.
2KJ+2H2O electrolysis J2+2H2+2KOH
Anode process.
A(+): 2J—2e-→J2
Process at the cathode.
K(-): 2H2O+2e-→H2+2OH-
As a result of electrolysis we observe:
When phenolphthalein is added to the near-cathode space, the solution becomes crimson in color, since the reduction of water molecules produces OH- ions, which create an alkaline environment.
When a starch solution is added to the near-anode space, we observe the appearance of a blue color, which is a qualitative reaction to molecular iodine, which is formed during the oxidation of J- ions.
Electrolysis of an aqueous solution of sodium sulfate.
- Na2SO4+2H2O electrolysis Na2SO4+2H2+O2↑
- 2H2O electrolysis 2H2+O2↑
Anode process.
A(+): H2O-4e-→O2+4H+
Process at the cathode.
K(-): 2H2O+2e-→H2+2OH-
When adding a solution of a universal indicator to the near-cathode space, we observe a blue color, since when water molecules are reduced, OH ions are formed, which give an alkaline environment.
When adding a solution of a universal indicator to the near-anode space, we observe a red color, since the oxidation of water molecules produces H+ ions, which give an acidic environment.
Sodium sulfate does not take part in electrolysis. Only water electrolysis occurs.
Electrolysis of an aqueous solution of copper (II) sulfate.
2CuSO4+2H2Oelectrolysis 2Cu+O2+2H2SO4
Anode process.
A(+): H2O-4e-→O2+4H+
Process at the cathode.
К(-): Cu2++2е-→Сu0
During the electrolysis of a solution of copper (II) sulfate at the cathode, we observe the release of a red copper precipitate.
Oxygen bubbles are released in the near-anode space.
Conclusion on the work done:
Electrolysis is a redox process that occurs at the electrodes when direct current is passed through a system including an electrolyte.
Electrolysis of solutions is complicated by the participation of H⁺ and OH⁻ ions in the electrode processes. In addition, water molecules themselves can undergo electrode oxidation or reduction.
Cathodic processes in aqueous solutions during electrolysis depend on the nature of the cation.
The processes occurring at the cathode depend on the oxidizing ability of the metal cation:
- Li, K, Ca, Na, Mg, Al Mn, Zn, Fe, Ni, Sn, Pb H Cu, Hg, Ag, Pt, Au
- Men⁺is not reduced (remains in solution)
- 2 Н₂О+ 2ē = Н₂↑+2 ОН⁻ Мen⁺ + nē = Me°
- 2 H₂O + 2ē = H₂↑ + 2 OH⁻ Men⁺ + nē = Me°
Anodic processes in aqueous solutions depend on the anode material and the nature of the anion.
Processes occurring at the anode
Oxygen-free acid residues
Oxygen-containing acid residues
- J⁻, Br⁻, S²⁻, Cl⁻ Oxidation of Am⁻ (except F⁻)
- Аm⁻ – m ē = A° OH⁻, SO₄²⁻, NO₃⁻, F⁻
In an alkaline environment:
- 4 OH⁻ – 4 ē = O₂↑ + 2 H₂O
- in acidic and neutral environments: 2 Н₂О – 4 ē = О₂↑ + 4 Н⁺
(We do not consider the influence of the anode material, since in laboratory work the influence of the anode material on the course of electrolysis is not considered).
8. Give the formulation of Faraday’s laws? What are their mathematical expressions? What is Faraday's number called? Compose electronic equations for the processes occurring on inert electrodes during the electrolysis of CdCl2 and CdSO4 solutions.
Answer:
The course of primary anodic and cathodic reactions during electrolysis is subject to Faraday's laws.
Faraday's first law: the mass of a substance m released at the electrode by an electric current is proportional to the amount of electricity Q passing through the electrolyte:
- m = kQ, but Q =It (1)
- where I – current strength, A; t – current transmission time, s.
- m = kIt (2)
k is a proportionality coefficient equal to the amount of substance released during the passage of one coulomb (C) of electricity (electrochemical equivalent).
Faraday's second law: the masses of different substances released by the same amount of electricity, proportional to their chemical equivalents (Me):
To release 1 gram equivalent of a substance, it is necessary to pass the same amount of electricity through the electrolyte, equal to approximately 96500 C (Faraday number). Hence:
Substituting the last equation into (2), we obtain a formula that combines both Faraday’s laws. (3)
Relationship (3) is used in calculations of processes during electrolysis.
Electrolysis of an aqueous solution of cadmium (II) chloride:
- CdCl2Cd2++2Cl-
- K(-):Cd2+, H2O A(+): Cl-, H2O
- Cd2++2e-→Cd 2Cl—2e-→Cl2
The overall electrolysis equation is:
CdCl2→Сd+Cl2
Electrolysis of an aqueous solution of cadmium (II) sulfate:
- CdSO4→ Cd2++SO42-
- K(-):Cd2+, H2O A(+):SO42-, H2O
- Cd2++2e-→Cd 2H2O-4e-→O2+4H+
The overall electrolysis equation is:
2CdSO4+2H2O→2Cd+O2+2H2SO4
Electrolysis in industry
The principle of separating substances using electricity is not complicated and has been well studied, so it is used in many places. As a result of decomposition, various chemical substances (metals, alkalis, gases) are obtained in their pure form, organic particles and inorganic material are synthesized, wastewater is purified, batteries are discharged, and the surfaces of equipment, tools and household items are protected from corrosion and other negative effects.
Electrolysis is the main (and often the only) way to obtain a substance of high purity and quality.
The use of electrolysis in technology, due to the ability of the cathode to deposit molecules of chemical elements on itself, makes it possible to create durable seamless pipes, reliable protective coatings for metal surfaces, jewelry, and precise casts of complex shapes.
Metal mining
Electrolysis of melts is effectively used in the separation of active metals such as aluminum, potassium, beryllium or sodium from ores or salts.
To create an electrolyte from bauxite - an aluminum ore in which the metal is in the form of an oxide - they are dissolved in cryolite.
In the container where electrolysis takes place, the cathode is the bottom covered with a layer of carbon, and the anode is an inert carbon rod. As a result of this reaction, the electrolysis product - pure aluminum - accumulates at the bottom and drains through special holes.
Electrometallurgy
The extraction of metals in electrometallurgy is carried out by two types of processes - electrothermal and electrochemical. In the first case, the separation of a pure substance or the formation of an alloy from ores and concentrates is achieved through the use of electricity as a source of thermal energy. In the second case, metal production is based on the principles of electrochemical interaction of substances.
Electrolysis of a melt or salt solution makes it possible to obtain metals of increased purity, separating them from impurities literally molecularly.
The refining process is the purification of copper
To obtain very pure, refined copper, rods or plates from already purified and with impurities of metal and electrolyte - a solution of copper sulfate - are used as electrodes.
The contaminated electrode becomes positively charged and, as an active anode, dissolves during the reaction.
Copper molecules are deposited on a clean cathode, and impurities fall to the bottom of the container in the form of sediment. Gold, silver and other non-ferrous metals are purified in the same way.
Electrotype
By applying a uniform metal layer to the original object, it is possible to create an unlimited number of its copies. To do this, an impression is taken from the original and covered with a layer of electrically conductive substance. This is how casts are made of complex surfaces, decorations, and much more.
Galvanopolishing
The principle of electroplating is based on the fact that when the original object in the electrolyte is exposed to electricity, the strongest electric field appears at the protrusions of its surface.
If it is positively charged, then during the electrolysis process it most quickly loses protruding electrons and in this way becomes smoother, that is, polished.
Anodizing
In this case, the protective coating of the metal surface is carried out using non-metallic compounds and sulfuric acid.
The metal part acts as an anode, and the electrolyte is acid.
During electrolysis, a decorative and protective layer of metal oxide is formed on the surface of the anode.
The scope of application of the technology is still wide; its principles have long been used even at home. But it is very important to carefully follow safety precautions.
Electroplating
By electrolysis, you can apply a thin uniform layer of metal to the surface of objects in order to make it less active, protect it from negative influences, decorate it, increase or decrease electrical conductivity.
In this case, the object being processed acts as an electrode, and the metal salt acts as an electrolyte.
Depending on the further purpose of the galvanized object, it is coated in this way with non-ferrous and precious metals.
Industrial use
Hall-Heroux process for aluminum production
- Electrometallurgy of aluminum, lithium, sodium, potassium, magnesium, calcium, and in some cases copper.
- The production of chlorine and sodium hydroxide, called the chlor-alkali process.
- Production of sodium chlorate and potassium chlorate.
- Production of perfluorinated organic compounds such as trifluoroacetic acid by electrofluorination.
- from refined copper.
- Production of fuels such as oxygen (for spacecraft and nuclear submarines) as well as hydrogen.
- and cleaning old coins and other metal objects.
Production processes
In manufacturing, electrolysis can be used to:
- Electroplating, in which a thin film of metal is deposited onto a substrate material. Electroplating is used in many industries for both functional and decorative purposes, such as car bodies and nickel coins.
- Electrochemical machining (ECM) in which an electrolytic cathode is used as a tool to remove material by anodic oxidation from the workpiece. ECM is often used as a method for deburring or etching metal surfaces such as tools or knives with a permanent mark or logo.
Electrophoresis
A therapeutic procedure using the principle of electrolysis is called electrophoresis. The drugs are injected directly into the skin so that they quickly enter the bloodstream and act. To do this, a material impregnated with an electrically conductive medicinal substance is laid between special electrodes and the human body. Charged particles, under the influence of electricity, penetrate the patient's skin and body.
The rate of penetration of the drug can be adjusted by varying the current strength.
Solid electrolytes: structure, application prospects
Currently, the most pressing issues are the efficiency and environmental safety of various technological processes. Great attention is paid to monitoring the state of the environment, synthesis and research of functional environmentally friendly materials with specified properties. Of great interest are solid electrolytes (ionic conductors), which can be used as oxygen-selective membrane materials in solid-phase fuel cells, oxygen pumps, exhaust gas analyzers for internal combustion engines, methane reforming, etc.
Solid electrolytes (ionic conductors, superionics) are solid-phase (crystalline, polycrystalline or amorphous - glassy) materials in which the ions of one of the sublattices have sufficiently high mobility, which determines conductivity values comparable to the characteristics of strong liquid electrolytes.
Unlike liquid electrolytes, solid electrolytes are substances intermediate in structure and properties between crystalline solids with a regular three-dimensional structure built from “stationary” atoms or ions, and liquid electrolytes that do not have a regular structure, but have mobile ions [1, 2].
Among the most promising structures that provide high ion transport, structures such as fluorite, perovskite and their derivatives are considered. High oxygen conductivity, combined with material stability in a wide range of oxygen partial pressure, was discovered in oxides with a perovskite structure based on heterosubstituted lanthanum gallate (La,Sr)(Ga,Mg)O3-y [3–5]. It was also found that additional modification of ion-conducting oxides based on lanthanum gallate with cations of transition elements provides high mixed-conducting characteristics of the oxides, which determines the prospects of these oxides for the development of electrodes for solid oxide fuel cells (SOFC) and the creation of oxygen-selective membranes for new methane conversion technology.
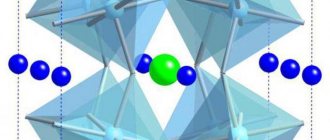
Oxides of the LAMOX family are also considered promising ionic conductors. These include lanthanum molybdates La2Mo2O9 [6] and their derivatives. -La2Mo2O9 is -SnWO4 [7] and is characterized by the presence of molybdenum-oxygen tetrahedra (Fig. 1).
The conductivity of lanthanum molybdate La2Mo2O9 and solid solutions based on it is comparable to the conductivity of stabilized zirconium oxide ZrO2/CaO (10-1-10-2 Ohm-1cm-1 at T = 1000oC) - the most widely used oxide electrolyte [8-9].
These objects must have a number of properties necessary for oxygen-ion conductivity. Such as a high concentration of anion vacancies (hopping mechanism of oxygen movement), high symmetry, ensuring equal potentials between occupied and vacant sites, high specific free volume (vacancies facilitate the diffusion of O2- ions), polarizable cations [10].
Studies of lanthanum molybdates using laser second harmonic generation (SHG) have shown that they have a non-centrosymmetric crystal structure and a phase transition between two different states of La2Mo2O9, corresponding to low- and high-temperature phases.
Studies of the SHG effect were carried out using the “reflection” scheme on fine powders and ceramics. The measurements were carried out using the relative method; quartz powder of the same dispersion as the powder sample under study was used as a standard.
The presence of the SHG signal of laser radiation (Fig. 2) indicates a non-centrosymmetric structure of lanthanum molybdate both at low and near 560oC with increasing temperature corresponds to a sharp decrease in the increase in the symmetry of the crystal structure of La2Mo2O9 to cubic, sp.gr. P213.
Fig. 2 Temperature dependence of the intensity of the second harmonic generation signal of La 2 Mo 2 O 9 .
In La2Mo2O9, the SHG intensity decreases rapidly with increasing temperature. This means that the noncentrosymmetric arrangement of the metal–oxygen bonds responsible for the SHG effect becomes more isotropic with temperature. In other words, oxygen ions, orderedly located at low temperatures in non-centrosymmetric crystallographic positions, with increasing temperature are distributed more evenly over a large number of positions, including centrosymmetric ones, which do not contribute to optical nonlinearity. Thus, the increase in the oxygen-ion conductivity of La2Mo2O9 with temperature in the cubic phase can be compared with a more uniform distribution of oxygen ions over a large number of -La2Mo2O9.
List of used literature:
- Ivanov-Shits Solid State Ionics. St. Petersburg University Publishing House, 2000. – 617 p.
- West A. Chemistry of solids, parts 1, 2. – M.: Mir, 1988. – 558 p.
- Ishihara T., Matsuda H., Takita Y. Doped LaGaO3 Perovskite Type Oxide as a New Oxide Ionic Conductor. // J. Amer. Chem. Soc. – V.116, No. 9. – P. 3801–3803.
- Politova E.D., Avetisov A.K., Zinnurov R.R., Kaleva G.M., Mordkovich V.Z., Mosunov A.V., Stefanovich S.Yu. Structure and electrical conductivity of (La9Sr0.1)[(Ga1xCrx)0.8Mg0.2]O3- solid solutions // Inorganic materials. -2006. t.42b No. 6. – pp. 760–766.
- PolitovaE.D., StefanovichS.Yu., AvetisovA.K., AleksandrovskiiV.V., GlavatskihT.Yu., GolubkoN.V., KalevaG.M., MosunovA.V., Venskovskii NU Processing, structure, microstructure and transport properties of the oxygen conducting ceramics (La,Sr)(Ga,M)Oy (M=Mg, Fe, Ni) // J. Solid State Electrochem. – 2004, v.8. No. 9. P. 655–660.
- Lacorre Ph. The LPS concept, a new way to look at anionic conductor // Solid State Sciences. 2000. V. 2. No. 3. P. 755–
- Ieischko W., Sleight AW Synthesis, properties and crystal structure of b-SnWO4 // Acta Crystallogr. V. 28. No. 11. P. 3174–3178.
- Khadasheva Z.S., Venskovsky N.U., Safronenko M.G., Mosunov A.V., Polittova E.D., Stefanovich S.Yu. Synthesis and properties of ionic oxygen conductors in the system La2(Mo1-XMX)2O9, (M=Nb, Ta) //Inorganic materials. - T. 38. N.11. – pp. 1381–1385.
- Khadasheva Z.S., Venskovsky N.U., Safronenko M.G. Mosunov A.V., Polittova E.D., Stefanovich S.Yu. Features of the preparation and properties of oxygen-conducting ceramics based on La2Mo2O9 // International Symposium “Phase transformations in solid solutions and alloys”. Sochi. – 2003. Collection of works. pp. 349–352.
Energy costs
The use of electrolysis requires high energy costs for several reasons:
- For the electrolysis process to be effective, it is necessary to ensure the presence of direct current to the electrodes for a long time;
- Often, the useful result of a reaction directly depends on the strength of the current - the higher the voltage, the faster and better it passes.
- Part of the energy is lost due to electrolyte resistance, anodic or cathodic overvoltage.
The course of the reaction, energy costs and benefits obtained can be predicted by knowing the basic laws of electrolysis.
Theoretical part
Electrolysis is a set of redox reactions occurring under the influence of direct electric current on electrodes immersed in a solution or molten electrolyte. During electrolysis, a chemical reaction is carried out due to the energy of an electric current supplied from the outside. Electrolysis is carried out in special devices - electrolyzers. This is a vessel with a solution or melt of an electrolyte and metal or graphite electrodes lowered into it. A potential difference from an external direct current source is applied to the electrodes. The cathode gives electrons to the particles of the substance in the electrolyte and reduces them. The anode takes electrons from particles in the electrolyte, oxidizing them.
Electrolysis of base melts.
During electrolysis, oxidation and reduction processes occur at various electrodes - anode and cathode. The anode is the electrode on which the oxidation process occurs. During electrolysis, the anode is positively charged. The cathode is the electrode at which the reduction process occurs. During electrolysis, the cathode is negatively charged. The redox processes occurring during electrolysis are influenced by various factors:
- Nature of electrolyte and solvent;
- Electrode material;
- Electrolysis mode (voltage, current, temperature).
It will be interesting All about Ohm's law: in simple words with examples for dummies
There are 2 types of electrolysis: melt electrolysis and electrolysis of electrolyte solutions. Electrolysis of oxide melts At the cathode, metal cations are reduced: Men++ nē = Me0, i.e. Metal is deposited at the cathode. Oxygen is oxidized at the anode: O –2 –2ē = O2 For example, electrolysis of molten potassium oxide: 2K2O = 4K + O2 When studying aluminum and methods for producing metals, you need to know the electrolysis of aluminum oxide. Metallic aluminum is produced by electrolysis of a solution of alumina Al2O3 in molten cryolite Na2AlF6 at 960–970°C. The electrolysis of Al2O3 can be represented by the following scheme: in the melt, aluminum oxide dissociates: Al2O3= Al3++ AlO3 3–, Al3+ ions are reduced at the cathode: Al3++3ē Al0, AlO3 ions 3– are oxidized at the anode: 4AlO3 3– – 12ē 2Al2O3 + 3O2. The overall equation of the process is: 2Al2O3 4Al + 3O2. Liquid aluminum collects at the bottom of the electrolyser.
Electrolysis of base melts
The metal is traditionally reduced at the cathode: Men+ +nē = Me0 At the anode, oxygen in the hydroxide group will be oxidized: 4OH− −4ē =2H2O + O2 Electrolysis of molten salts 1. Electrolysis of a molten oxygen-free salt: Metal is always reduced at the cathode: Men + nē = Me0 An oxygen-free anion is oxidized at the anode: A n– – nē = A0 For example: Electrolysis of a NaCl melt: 2NaCl = 2Na + Cl2 2. Electrolysis of a melt of oxygen-containing salt (the anion element is not in the highest oxidation state): The metal is always reduced at the cathode: Men++ nē = Me0 The anion element will be oxidized at the anode: SO3 2– – 2ē = SO3 0 For example, electrolysis of molten sodium sulfite: Na2SO3 = 2Na + SO3 Sulfur S in sulfite has an oxidation state of +4, during electrolysis it is oxidized to +6 (SO3). 3.
Electrolysis of a molten oxygen-containing salt (anion element in the highest oxidation state): Metal is always reduced at the cathode: Men++ nē = Me0 At the anode: because
element is already in the highest degree of oxidation, then oxygen will be oxidized, for example: 2CO3 –2 – 4ē = 2CO2 + O2 For example, electrolysis of a molten sodium carbonate: 2Na2CO3 = 4Na + 2CO2+ O2 It is important to understand that these reactions do not occur on their own. Their occurrence is possible only under the influence of electric current.
Electrolysis of solutions The following reduction reactions can occur at the cathode.
Electrolysis of base melts.
How does electrolysis work?
Safety precautions when working with acids
Most acids, especially in the form of concentrated solutions, cause severe burns and destroy body tissue. That’s why it’s so important to follow safety precautions when working with them.
- Be sure to work carefully, without spilling anything on yourself or nearby objects.
- If acid gets on your hands, wash with plenty of running water, then with a solution of baking soda.
- Also, with a weak solution of baking soda, you should treat the surface of furniture or other household items made of wood, metal, or plastic as quickly as possible if acid comes into contact with them.
- If it gets into your eyes, also rinse with plenty of water and be sure to go to the doctor.
- Store these dangerous substances in tightly closed packaging, in places inaccessible not only to children, but also to pets.