The efficient and durable operation of electrical machines and installations directly depends on the state of the insulation for which electrical materials are used. They are characterized by a set of certain properties when placed in an electromagnetic field, and are installed in devices taking these indicators into account.
The classification of electrical materials allows us to divide into separate groups electrical insulating, semiconductor, conductor and magnetic materials, which are complemented by basic products: capacitors, wires, insulators and finished semiconductor elements.
Materials operate both in separate magnetic or electric fields with certain properties, and are exposed to several radiations simultaneously. Magnetic materials are conventionally divided into magnetic and weakly magnetic substances. Highly magnetic materials are most widely used in electrical engineering.
Materials Science
A material is a substance characterized by a different chemical composition, properties and structure of molecules and atoms from other objects. Matter is in one of four states: gaseous, solid, plasma or liquid. Electrical and structural materials perform various functions in an installation.
Conducting materials transmit the flow of electrons, dielectric components provide insulation. The use of resistive elements converts electrical energy into thermal energy; structural materials retain the shape of the product, for example, the housing. Electrical and structural materials necessarily perform not one, but several related functions, for example, the dielectric experiences loads during the operation of an electrical installation, which brings it closer to structural materials.
Electrical materials science is a science that deals with determining the properties and studying the behavior of a substance under the influence of electricity, heat, frost, magnetic field, etc. Science studies the specific characteristics necessary for the creation of electrical machines, instruments and installations.
Classification of materials according to electrical properties
During the manufacturing process and under various operating conditions, electrical materials are exposed to electric and magnetic fields separately and together. Based on their behavior in an electric field, these materials are divided into conductor, semiconductor and dielectric.
The classification of electrical materials by electrical properties is based on the concepts of the band theory of electrical conductivity of solids. The essence of this theory is as follows.
In an isolated atom, electrons revolve around the nucleus in certain orbits. Each orbit can contain no more than two electrons. Each orbit corresponds to a strictly defined energy value that an electron can possess, i.e., each orbit represents a certain energy level.
Under the influence of the attraction of a positively charged atomic nucleus, electrons tend to occupy the levels closest to the nucleus with the minimum energy value. Therefore, the lower energy levels turn out to be filled with electrons, and the upper levels are free. An electron can jump from the lower energy level W1 to another free level W2 (Fig. 2.1). To do this, the electron must be given additional energy. If there are no free levels in the atom, then the electron cannot change its energy, and therefore does not participate in the creation of electrical conductivity.
In a crystal lattice consisting of several atoms, individual energy levels are split into sublevels, which form energy zones (see Fig. 2.1). In this case, free and filled energy levels are split. The band filled with electrons is called valence.
The upper level of the valence band is designated Wv.
The free zone is called the conduction
The lower level of the conduction band is designated Wc
.
The gap between the valence band and the conduction band is called
the band
gap W. The value of the band gap significantly affects the properties of materials.
Figure 2.11 Energy level diagram of an isolated atom (1) and a solid (2).
If W is equal to or close to zero, then electrons can move to free levels due to their own thermal energy and increase the conductivity of the substance. Substances with such a structure of energy zones are classified as conductors.
Typical conductors are metals.
If the value of the bandgap exceeds several electron volts (1 eV is the energy an electron receives when moving between two points of an electric field with a potential difference of 1 V), then significant energy is required for the transition of electrons from the valence band to the conduction band. Such substances are classified as dielectrics.
Dielectrics have high electrical resistivity.
If the exclusion zone value is 0.1. 0.3 eV, then electrons easily move from the valence band to the conduction band due to external energy. Substances with controlled conductivity are classified as semiconductors.
Conducting materials serve to conduct electric current.
Typically, conductors include substances with electrical resistivity p
less than Ohm*m.
Dielectric materials have the ability to block the passage of current.
Dielectric materials include substances with electrical resistivity p
more than 10 7 Ohm*m. Due to their high electrical resistivity, they are used as electrical insulating materials.
Depending on the structure and external conditions, materials can move from one class to another. For example, solid and liquid metals are conductors, and metal pairs are dielectrics; germanium and silicon, typical semiconductors under normal conditions, become conductors when exposed to high hydrostatic pressures; carbon in the diamond modification is a dielectric, and in the graphite modification it is a conductor.
Semiconductor materials have conductivity, which can be used to control voltage, temperature, light, etc.
The electrical resistivity of semiconductors is Ohm*m.
The main property of a substance in relation to an electric field is electrical conductivity.
Electrical conductivity is characterized by specific electrical conductivity and specific electrical resistance p
:
(2)
J—
current density;
y
—electrical conductivity, S/m;
Eelectric field strength, V/m; p
= 1 /
y
- electrical resistivity, Ohm-m.
Specific electrical conductivity values for
and electrical resistivity
p
vary significantly between different materials. In a superconducting state, the electrical resistivity of materials is zero, and in rarefied gases it tends to infinity.
Date added: 2015-06-17; ; ORDER A WORK WRITING
Conductors
These include electrical materials, the main indicator of which is the pronounced conductivity of electric current. This happens because the mass of the substance constantly contains electrons that are weakly bound to the nucleus and are free charge carriers. They move from the orbit of one molecule to another and create a current. The main conductor materials are copper and aluminum.
Conductors include elements that have a specific electrical resistance of ρ < 10-5, while an excellent conductor is a material with an indicator of 10-8 Ohm*m. All metals conduct current well; of the 105 elements in the table, only 25 are not metals, and of this heterogeneous group, 12 materials conduct electric current and are considered semiconductors.
The physics of electrical materials allows their use as conductors in gaseous and liquid states. As a liquid metal with normal temperature, only mercury is used, for which this is a natural state. Other metals are used as liquid conductors only in a heated state. Conducting liquids, such as electrolyte, are also used for conductors. The important properties of conductors, which make it possible to distinguish them by the degree of electrical conductivity, are the characteristics of thermal conductivity and the ability to generate thermal energy.
Asbestos materials
Asbestos is a natural mineral that has a fibrous structure. A qualitative indicator of asbestos is its high heat resistance (300 – 400°C) and low thermal conductivity. Materials are made from asbestos in the form of sheets of different thicknesses in the form of ropes of different diameters and asbestos fabrics. Asbestos has poor electrical insulating properties (dielectric strength 0.6 - 1.2 kV/mm). Most often, asbestos is used as a heat insulator. It is used as an electrical insulator only in low-voltage installations.
Dielectric materials
Unlike conductors, the mass of dielectrics contains a small number of free electrons of elongated shape. The main property of a substance is its ability to obtain polarity under the influence of an electric field. This phenomenon is explained by the fact that under the influence of electricity, bound charges move towards the acting forces. The higher the electric field strength, the greater the displacement distance.
Insulating electrical materials are the closer to ideal, the lower the specific conductivity, and the less pronounced the degree of polarization, which allows us to judge the dissipation and release of thermal energy. The conductivity of a dielectric is based on the action of a small number of free dipoles that shift in the direction of the field. After polarization, the dielectric forms a substance with different polarities, that is, two different signs of charges are formed on the surface.
The use of dielectrics is most extensive in electrical engineering, since the active and passive characteristics of the element are used.
Active materials with controllable properties include:
- pyroelectrics;
- electroluminescent phosphors;
- piezoelectrics;
- Ferroelectrics;
- electrets;
- materials for laser emitters.
The main electrical materials are dielectrics with passive properties, used as insulating materials and conventional capacitors. They are able to separate two sections of an electrical circuit from one another and prevent the flow of electrical charges. With their help, live parts are insulated so that electrical energy does not go into the ground or onto the housing.
Electrical insulating varnished fabrics
Lakotkany and fiberglass fabrics are flexible materials and are made from cotton, glass or silk fabric. After this, the fabric is impregnated with oil-bitumen or oil varnish or other insulating composition. They are produced in rolls with a thickness of 0.1-0.3 mm and a width of 700 to 1000 mm. Brands of varnished fabric produced by the industry: LHS, LKHSM, LKHSS, LHCh, LShS. Fiberglass grades LSB, LSM, LSE, LSMM, LSK, LSKR, LSKL. fabric of the LShS brand is also produced with a thickness of 0.08 mm, and LShSS can have a thickness of 0.04 mm.
Lakotkan
For brands of varnished fabrics and glass fabrics, the abbreviation in the name is deciphered as follows: L - varnished fabric; X - cotton; C - in second place - glass; K - in second place - nylon; C - in third place - light; K - in third place - organosilicon; C - in fourth place - special; L - in fourth place - sticky; Ch - black; Ш - silk; B - bitumen-oil-alkyd; M - oil resistant; R - rubber; E - escapone. Fiberglass fabric has high heat resistance. Brands LSKL and LSK - about 180°C, and brand LBS reaches 130°C. Their electrical strength is 35 - 40 kV/mm.
Fiberglass
Lacquered fabric and fiberglass are used as electrical and thermal insulating materials. Most often, they insulate layers of coil windings.
Dielectric separation
Dielectrics are divided into organic and inorganic materials, depending on their chemical composition. Inorganic dielectrics do not contain carbon, while organic forms have carbon as their main element. Inorganic substances such as ceramics and mica have a high degree of heating.
Based on the method of production, electrical materials are divided into natural and artificial dielectrics. The widespread use of synthetic materials is based on the fact that manufacturing makes it possible to impart specified properties to the material.
Based on the structure of molecules and molecular lattice, dielectrics are divided into polar and non-polar. The latter are also called neutral. The difference is that atoms and molecules, before the action of electric current on them, have or do not have an electric charge. The neutral group includes fluoroplastic, polyethylene, mica, quartz, etc. Polar dielectrics consist of molecules with a positive or negative charge, an example is polyvinyl chloride, bakelite.
Electrical insulating materials and areas of their application
The main areas of application of electrical insulating materials include various industrial branches, radio engineering, instrument making and installation of electrical networks. Dielectrics are the main elements on which the safety and stability of any electrical appliance depends. The quality and functionality of insulation is influenced by various parameters.
Thus, the main reason for using electrical insulation is to comply with safety regulations. In accordance with them, it is strictly prohibited to operate equipment with partially or completely missing insulation or damaged shell, since even small currents can harm the human body.
Properties of dielectrics
Based on their state, dielectrics are divided into gaseous, liquid and solid. Solid electrical materials are the most commonly used. Their properties and application are assessed using indicators and characteristics:
- volumetric resistivity;
- the dielectric constant;
- surface resistivity;
- thermal permeability coefficient;
- dielectric losses expressed by the tangent of the angle;
- strength of the material under the influence of electricity.
Volume resistivity depends on the ability of a material to resist the flow of constant current through it. The inverse of resistivity is called volumetric conductivity.
Surface resistivity is determined by the ability of a material to resist direct current flowing across its surface. Surface conductivity is the reciprocal of the previous indicator.
The coefficient of thermal permeability reflects the degree of change in resistivity after increasing the temperature of a substance. Typically, as the temperature increases, the resistance decreases, therefore, the coefficient value becomes negative.
Dielectric constant determines the application of electrical materials in accordance with the ability of the material to create electrical capacity. The indicator of the relative permeability of a dielectric is included in the concept of absolute permeability. The change in insulation capacity is shown by the previous indicator of the thermal permeability coefficient, which simultaneously shows an increase or decrease in capacity with a change in temperature.
The dielectric loss tangent reflects the degree of power loss of the circuit relative to the dielectric material exposed to electrical alternating current.
Electrical materials are characterized by an indicator of electrical strength, which determines the possibility of destruction of a substance under the influence of voltage. When identifying mechanical strength, there are a number of tests to establish the ultimate strength in compression, tension, bending, torsion, impact and splitting.
Electrical properties and material characteristics (general)
Electric and magnetic fields do not exist separately (independently), because They give birth to each other. Electrical materials
Electrical materials are materials that have certain properties in relation to the electromagnetic field and are used in technology taking these properties into account (various materials are exposed to both individual electric and magnetic fields and their combination).
Application: electrical machines, devices, instruments and other elements of electrical equipment and electrical installations.
Classification of electrical materials.
1. In an electric field.
1. Conductor materials (conductors) are materials in which an electric current arises under the influence of an electric field (metals and their alloys, graphite).
Conductors contain free charge carriers and under the influence of an electric field they acquire directional movement. This ordered movement of electric charges is electric current.
Application: live parts of electrical machines, devices and networks.
2. Semiconductor materials (semiconductors) are materials in which an electric current arises under the influence of an eclectic field, but their conductivity depends on external conditions (temperature, impurities, light, electric and magnetic fields, pressure, nuclear radiation, etc.) (germanium Ge, silicon Si, silicon carbide SiC).
Application: electronic equipment (diodes, transistors, thyristors).
3. D- electric materials (dielectrics) are materials that, under the influence of an electric field, do not create an electric current under normal conditions, the main electrical property of which is the ability to polarize in an electric field (rubber, plastics, glass).
In dielectrics, electric charges are firmly bound to atoms, molecules or ions and in an electric field they can only shift, and the centers of positive and negative charges separate, i.e. polarization.
Application: insulation of live parts from each other, surrounding objects and personnel.
2. In a magnetic field.
1. Weakly magnetic materials are materials whose magnetic susceptibility is very low (copper Cu, aluminum Al, lead Pb, organic compounds).
Application: not widely used in technology.
2. Highly magnetic materials (magnets) are materials that are magnetized under the influence of a magnetic field and thereby strengthen it (iron Fe, nickel Ni, cobalt Co and their alloys).
Application: cores and magnetic circuits of electrical machines and devices, permanent magnets.
MECHANICAL PROPERTIES AND CHARACTERISTICS OF MATERIALS
Mechanical characteristics make it possible to evaluate the ability of materials to withstand external static and dynamic loads; they are necessary for selecting technological processing of materials (cutting, stamping, casting), strength calculations, monitoring and diagnosing the condition of structural parts during operation.
Tensile testing is carried out on cylindrical samples and bars with a rectangular cross-section. The sample is secured at its ends in the grips of the testing machine. The lower grip is stationary; a destructive tensile force is applied to the other, which is gradually increased at a certain speed until the sample breaks.
1. Plasticity is the property of a material to irreversibly change its shape and size under the influence of external mechanical loads.
where ∆lorest is the increment in sample length after rupture, mm;
l0 – initial sample length, mm.
The greater the elongation value, the more ductile the material.
2. Strength is the property of a material to resist deformation or destruction under the influence of external mechanical loads.
Breaking tensile stress (tensile strength)
where Рр – failure load upon rupture of the sample, N;
S0 – cross-sectional area of the sample before testing, mm2.
The higher the tensile strength value, the stronger the material.
3. Hardness is the property of a material to resist penetration of a harder body (indenter) into its surface.
An indenter is a carbide tip in the form of a ball, pyramid or cone, the hardness of which significantly exceeds the hardness of the material being tested.
Using the Brinell method, a steel ball is pressed into the surface of the material.
where P is the load on the indenter, N;
Stp – print surface area, mm2.
According to the Vickers method, a diamond tetrahedral pyramid is pressed into the surface of the material under the influence of a load.
The higher the hardness value, the harder the material.
4. Impact strength is the property of a material to resist impact loads.
The impact bending test is carried out on bars with a rectangular cross-section (for metals with a U-shaped and V-shaped notch). The sample is placed in a pendulum impact driver. The impact applied to the center of the sample by the pendulum is gradually increased. The pointer on the scale of the pile driver records the value of the work expended by the pendulum to destroy the sample.
where ∆A is the work expended by the pendulum to destroy the sample, MJ.
The higher the impact strength value, the less brittle the material is.
Electrical properties and material characteristics (general)
Electrical characteristics make it possible to evaluate the properties of materials when exposed to an electric field. The main property of electrical materials in relation to the electric field is electrical conductivity.
Electrical conductivity is the property of a material to conduct electric current under the influence of a constant (not changing over time) electrical voltage.
1. Electrical resistivity is the resistance of a material 1 m long and with a cross section of 1 m2.
where γ is the specific conductivity of the material, this is the conductivity of a material with a length of 1 m and a cross section of 1 m2, 1/Ohm∙m;
q – value of the charge of the carrier (electron charge 1.6·10-19), C;
n is the number of charge carriers per unit volume;
µ – charge carrier mobility.
The higher the ρ value, the lower the electrical conductivity of the material.
Conductor resistance is a design characteristic of a conductor, because depends on the size and conductive properties of the material.
where ρ is the resistivity of the material, Ohm∙m;
l – conductor length, m;
S – cross-sectional area of the conductor, m2.
2. Temperature coefficient of resistivity - shows how much the resistance of a material in 1 Ohm will change when it is heated by 1 0C.
With a linear change in resistivity in a narrow temperature range
where ρ is the resistivity of the material at temperature;
ρ0 – resistivity of the material at initial
temperature t0, usually taken to be 200C.
If we replace resistivity with resistance
The larger the value of α, the more the resistance of the conductor changes with temperature changes.
Conductors α>0 with increasing temperature, the resistivity of the material increases.
Semiconductors and dielectrics α
Physical and chemical properties of dielectrics
Dielectrics contain a certain number of released acids. The amount of potassium hydroxide in milligrams required to get rid of impurities in 1 g of the substance is called the acid number. Acids destroy organic materials and have a negative effect on insulating properties.
The characteristics of electrical materials are supplemented by a viscosity or friction coefficient, indicating the degree of fluidity of the substance. Viscosity is divided into conditional and kinematic.
The degree of water absorption is determined depending on the mass of water absorbed by an element of the test size after being in water for a day at a given temperature. This characteristic indicates the porosity of the material; an increase in the indicator worsens the insulating properties.
Magnetic materials
Indicators for assessing magnetic properties are called magnetic characteristics:
- magnetic absolute permeability;
- magnetic relative permeability;
- thermal magnetic permeability coefficient;
- energy of the maximum magnetic field.
Magnetic materials are divided into hard and soft. Soft elements are characterized by small losses when the magnetization of the body lags behind the acting magnetic field. They are more permeable to magnetic waves, have a low coercive force and increased inductive saturation. They are used in the construction of transformers, electromagnetic machines and mechanisms, magnetic screens and other devices where magnetization with low energy losses is required. These include pure electrolytic iron, Armco iron, permalloy, electrical steel sheets, and nickel-iron alloys.
Solid materials are characterized by significant losses when the degree of magnetization lags behind the external magnetic field. Having received magnetic pulses once, such electrical materials and products are magnetized and retain the accumulated energy for a long time. They have high coercive force and high residual induction capacity. Elements with such characteristics are used to make stationary magnets. Representative elements are iron-based alloys, aluminum, nickel, cobalt, and silicon components.
Auxiliary equipment
This category of goods includes various auxiliary equipment that ensures the uninterrupted operation of household appliances and industrial equipment. This category is no less in demand and not a single modern installation can do without it. The most common auxiliary devices are:
- stabilizers that protect consumer devices from power surges;
- uninterruptible power supplies that allow equipment to continue operating for some time during a power outage. Many modern models also allow you to equalize fluctuations in the network.
- consumables – include batteries, chargers and other products.
Area of use of ferromagnets
They are used most effectively to create transformer coil cores. The use of the material makes it possible to significantly increase the magnetic field of the transformer, without changing the current readings. Such ferrite inserts allow you to save electricity consumption when operating the device. Electrical materials and equipment, after turning off the external magnetic influence, retain magnetic indicators and maintain the field in the adjacent space.
Elementary currents do not pass after the magnet is turned off, thus creating a standard permanent magnet that works effectively in headphones, telephones, measuring instruments, compasses, and sound recording devices. Permanent magnets that do not conduct electricity are very popular in use. They are obtained by combining iron oxides with various other oxides. Magnetic iron ore belongs to ferrites.
Protective devices
In the modern world, the demand for electrical products increases every year, and new equipment comes to replace outdated equipment. Without such devices, the operation of the electrical network is impossible. In addition, they ensure operational safety and increase the service life of household appliances and industrial equipment.
Also, such products allow you to automate some processes. An example is circuit breakers that conduct circuit current in normal modes and automatically protect electrical networks and equipment from emergency modes.
The residual current device is also important. It turns off the system in the event of a current leak as a result of a breakdown on the housing of electric heaters, ovens, washing machines and other household appliances, and thus protects a person from electric shock.
One of the most modern protective devices are differential circuit breakers, which combine the functions of a circuit breaker and an RCD.
Semiconductor materials
These are elements that have a conductivity value that is in the range of this indicator for conductors and dielectrics. The conductivity of these materials directly depends on the appearance of impurities in the mass, external directions of influence and internal defects.
The characteristics of electrical materials of the semiconductor group indicate a significant difference between the elements from each other in structural lattice, composition, and properties. Depending on the specified parameters, materials are divided into 4 types:
- Elements containing atoms of the same type: silicon, phosphorus, boron, selenium, indium, germanium, gallium, etc.
- Materials containing metal oxides - copper, cadmium oxide, zinc oxide, etc.
- Materials grouped into the antimonide group.
- Organic materials – naphthalene, anthracene, etc.
Depending on the crystal lattice, semiconductors are divided into polycrystalline materials and monocrystalline elements. The characteristics of electrical materials make it possible to divide them into non-magnetic and weakly magnetic. Magnetic components include semiconductors, conductors, and non-conductors. A clear distribution is difficult to achieve, since many materials behave differently under changing conditions. For example, the performance of some semiconductors at low temperatures can be compared to the performance of insulators. The same dielectrics, when heated, act like semiconductors.
Classification of materials according to electrical properties
All materials, depending on their electrical properties, can be divided into dielectrics
,
conductors
and
semiconductors
. The difference between dielectrics, conductors and semiconductors can be most clearly shown using energy diagrams of the band theory of solids [2]. In the energy diagram of a solid, three zones are distinguished: filled with electrons, forbidden (electrons of a given material cannot have such energies) and conduction zone (free zone) (Fig. 1).
At the dielectric
the forbidden zone is so large (
3.5 eV), that free electrons practically do not appear and electrons are not observed under normal conditions, since the energy
Only photons of cosmic rays and radioactive radiation have 3.5 eV.
Semiconductors
have a narrow band gap (3.5 > 1).
Weakly magnetic materials are rarely used in technology, so we will not consider them. In the energy sector, only materials with µ >> 1 are used as magnetic materials.
Thus, in the section “Electrical materials” the following groups of materials will be considered:
magnetic materials (µ >> 1).
Construction materials
– solid materials intended for the manufacture of products subject to mechanical loading.
They are divided into types, the main ones being:
− metals and alloys;
− non-metallic materials (plastics, polymers, wood, etc.);
Composite materials
Materials that are divided not by functioning, but by composition, are called composite materials; these are also electrical materials. Their properties and application are determined by the combination of materials used in their manufacture. Examples include sheet glass fiber components, fiberglass, and mixtures of electrically conductive and refractory metals. The use of equivalent mixtures allows us to identify the strengths of the material and use them for their intended purpose. Sometimes a combination of composite components leads to the creation of a completely new element with different properties.
Gaseous dielectrics
The most common gaseous dielectrics are air, nitrogen, hydrogen and SF6 gas. Electrical insulating gases are divided into natural and artificial. Natural air includes air, which is used as insulation between live parts of power lines and electrical machines. As an insulator, air has disadvantages that make it impossible to use in sealed devices. Due to the presence of a high concentration of oxygen, air is an oxidizing agent, and in inhomogeneous fields the low electrical strength of air appears.
Nitrogen is used as insulation in power transformers and high-voltage cables. Hydrogen, in addition to being an electrical insulating material, is also a forced cooling agent, so it is often used in electrical machines. In sealed installations, SF6 gas is most often used. Filling with SF6 gas makes the device explosion-proof. It is used in high-voltage circuit breakers due to its arc-extinguishing properties.
Film materials
Films and tapes as electrical materials have gained a wide range of applications in electrical engineering. Their properties differ from other dielectrics in flexibility, sufficient mechanical strength and excellent insulating characteristics. The thickness of the products varies depending on the material:
- films are made with a thickness of 6-255 microns, tapes are produced with a thickness of 0.2-3.1 mm;
- polystyrene products in the form of tapes and films are produced with a thickness of 20-110 microns;
- polyethylene tapes are made with a thickness of 35-200 microns, a width from 250 to 1500 mm;
- fluoroplastic films are made with a thickness of 5 to 40 microns, the width is 10-210 mm.
The classification of electrical materials made from films allows us to distinguish two types: oriented and non-oriented films. The first material is used most often.
Insulation materials
The following requirements are imposed on electrical insulating materials used in electrical machines: high electrical strength, mechanical strength, heat resistance and thermal conductivity, as well as low hygroscopicity. It is important that the insulation be as thin as possible, since an increase in the thickness of the insulation impairs heat transfer and leads to a decrease in the fill factor of the groove with conductor material, which in turn causes a decrease in the rated power of the machine. In some cases, other requirements also arise, for example, resistance against various microorganisms in humid tropical climates, and so on. In practice, all these requirements can be satisfied to varying degrees.
Insulating materials can be solid, liquid or gaseous. The gases are usually air and hydrogen, which represent an ambient or cooling medium in relation to the machine and at the same time, in some cases, play the role of electrical insulation. Liquid dielectrics are used mainly in transformer manufacturing in the form of a special type of mineral oil called transformer oil.
Solid insulating materials are of greatest importance in electrical engineering. They can be divided into the following groups: 1) natural organic fibrous materials - cotton paper, wood pulp-based materials and silk; 2) inorganic materials - mica, fiberglass, asbestos; 3) various synthetic materials in the form of resins, films, sheet material, and so on; 4) various enamels, varnishes and compounds based on natural and synthetic materials. In recent years, organic fiber insulation materials have been increasingly replaced by synthetic materials.
Enamels are used for insulating wires and as outer insulation for windings. Varnishes are used for gluing layered insulation and for impregnating windings, as well as for applying a protective coating layer to the insulation. By impregnating the windings two or three times with varnishes, alternating with drying, the pores in the insulation are filled, which increases the thermal conductivity and electrical strength of the insulation, reduces its hygroscopicity and mechanically holds the insulation elements together.
Impregnation with compounds serves the same purpose as impregnation with varnishes. The only difference is that the compounds do not have volatile solvents, but are a very consistent mass, which, when heated, softens, liquefies and is capable of penetrating into the pores of the insulation under pressure. Due to the absence of solvents, the filling of pores during compounding is more dense. The most important characteristic of insulating materials is their heat resistance, which decisively affects the reliability of operation and service life of electrical machines. According to heat resistance, electrical insulating materials used in electrical machines and devices are divided, according to GOST 8865-70, into seven classes with the following maximum permissible temperatures ϑmax:
| Insulation class | Y | A | E | B | F | H | C |
| ϑmax, °C | 90 | 105 | 120 | 130 | 155 | 180 | >180 |
The standards of previous years contain the old designations of some insulation classes: instead of Y, E, F, H, respectively, O, AB, BC, SV.
Class Y includes fibrous materials made of cotton paper, cellulose and silk that are not impregnated with liquid dielectrics or immersed in them, as well as a number of synthetic polymers (polyethylene, polystyrene, polyvinyl chloride, etc.). This insulation class is rarely used in electrical machines.
Class A includes fibrous materials made of cotton paper, cellulose and silk, impregnated or immersed in liquid electrical insulating materials, insulation of enamel wires based on oil and polyamide resole varnishes (nylon), polyamide films, butyl rubber and other materials, as well as impregnated wood and wood laminates. Impregnating substances for this class of insulation are transformer oil, oil and asphalt varnishes and other substances with appropriate heat resistance. This class includes various varnished fabrics, tapes, electrical cardboard, getinaks, textolite and other insulating products. Class A insulation is widely used for rotating electrical machines with power up to 100 kW and above, as well as in the transformer industry.
Class E includes insulation of enamel wires and electrical insulation based on polyvinyl acetal (viniflex, metalvin), polyurethane, epoxy, polyester (lavsan) resins and other synthetic materials with similar heat resistance. Insulation class E includes new synthetic materials, the use of which is rapidly expanding in low and medium power machines (up to 10 kW and above).
Class B combines insulating materials based on inorganic dielectrics (mica, asbestos, fiberglass) and adhesive, impregnating and coating varnishes and resins of increased heat resistance of organic origin, and the content of organic substances by weight should not exceed 50%. This includes, first of all, materials based on thin plucked mica (micalenta, micafolia, micanite), widely used in electrical engineering.
Recently, mica materials have also been used, which are based on a continuous mica ribbon of mica plates up to several millimeters in size and several microns thick.
Class B also includes various synthetic materials: polyester resins based on phthalic anhydride, polychlorotrifluoroethylene (fluoroplastic-3), some polyurethane resins, plastics with inorganic filler, etc.
Class F insulation includes materials based on mica, asbestos and fiberglass, but with the use of organic varnishes and resins modified with organosilicon (organosiloxane) and other resins with high heat resistance, or with the use of other synthetic resins of corresponding heat resistance (polyester resins based on ISO - and terephthalic acids, etc.). Insulation of this class must not contain cotton, cellulose or silk.
Class H includes insulation based on mica, fiberglass and asbestos in combination with organosilicon (organopolysiloxane), polyorganometallosilxane and other heat-resistant resins. Using such resins, micanites and mica, as well as steklomicanites, steklomicafolium, steklomicalents, steklosludinit, glass laminates and fiberglass laminates are produced.
Class H also includes insulation based on polytetrafluoroethylene (PTFE-4). Class H materials are used in electrical machines operating in very difficult conditions (mining and metallurgical industries, transport installations, etc.).
Class C insulation includes mica, quartz, fiberglass, glass, porcelain and other ceramic materials used without organic binders or with inorganic binders.
Under the influence of heat, vibration and other physical and chemical factors, the insulation ages, i.e., it gradually loses its mechanical strength and insulating properties. It has been experimentally established that the service life of class A and B insulation is reduced by half with an increase in temperature of every 8-10° above 100°C. Similarly, the service life of other classes of insulation also decreases with increasing temperature.
Varnishes and enamels for electrical insulation
Solutions of substances that form a film when hardened are modern electrical materials. This group includes bitumen, drying oils, resins, cellulose ethers or compounds and combinations of these components. The transformation of the viscous component into an insulator occurs after evaporation from the mass of the applied solvent and the formation of a dense film. Based on the method of application, films are divided into adhesive, impregnating and covering.
Impregnating varnishes are used for windings of electrical installations in order to increase the coefficient of thermal conductivity and moisture resistance. Covering varnishes create a top protective coating against moisture, frost, oil for the surface of windings, plastics, and insulation. Adhesive components are capable of gluing mica plates to other materials.
Liquid dielectrics
Liquid electrical insulating materials are often used in electrical machines and apparatus. In a transformer, oil plays the role of insulation. Liquid dielectrics also include liquefied gases, unsaturated vaseline and paraffin oils, polyorganosiloxanes, distilled water (purified from salts and impurities).
The main characteristics of liquid dielectrics are dielectric constant, electrical strength and electrical conductivity. Also, the electrical parameters of dielectrics largely depend on the degree of their purification. Solid impurities can increase the electrical conductivity of liquids due to the proliferation of free ions and electrons. Purification of liquids by distillation, ion exchange, etc. leads to an increase in the electrical strength of the material, thereby reducing its electrical conductivity.
Liquid dielectrics are divided into three groups:
- petroleum oils;
- vegetable oils;
- synthetic fluids.
The most commonly used petroleum oils are transformer, cable and capacitor oils. Synthetic fluids (organosilicon and organofluorine compounds) are also used in apparatus construction. For example, organosilicon compounds are frost-resistant and hygroscopic, therefore they are used as an insulator in small transformers, but their cost is higher than the price of petroleum oils.
Vegetable oils are practically not used as insulating materials in electrical insulating technology. These include castor oil, flaxseed oil, hemp oil and tung oil. These materials are low-polarity dielectrics and are used mainly for impregnation of paper capacitors and as a film-forming substance in electrical insulating varnishes, paints, and enamels.
Compounds for electrical insulation
These materials appear as a liquid solution at the time of use, followed by hardening and hardening. The substances are characterized by the fact that they do not contain solvents. Compounds also belong to the group “electrical materials”. Their types are pouring and impregnating. The first type is used to fill cavities in cable couplings, and the second group is used to impregnate motor windings.
Compounds are produced as thermoplastic, they soften after increasing temperatures, and thermosetting, which firmly retain their hardening shape.
Cotton ribbons
The industry produces cotton ribbons of the following varieties: keeper, taffeta, cambric and calico. Tapes are produced in the following types and sizes:
- Keeper tape LE is made according to GOST 4514-78 from cotton thread and has a width of 10-60 mm and a thickness of 0.45 mm, used in electrical installation work, for tightening cables and wires, for tying coils, windings of motors and transformers;
- Taffeta ribbon LE is made according to GOST 4514-78 from cotton or silk thread and has a width of 10-50 mm in increments of 5 mm, and a thickness of 0.25 mm, used for electrical installation work. It is similar to keeper tape, differing only in the weaving of the thread. In terms of strength characteristics it is inferior to keeper tape.
- The cambric tape LE is made according to GOST 4514-78 from cotton plain weave thread, has a width of 10-20 mm and a thickness of 0.12-0.16-0.18 mm. The thinnest of the ribbons. Can be replaced with taffeta.
- Calico tape LE are manufactured according to GOST 4514-78, have a width of 12-35 mm and a thickness of 0.22 mm. In terms of physical properties, it is less durable than kiper, but stronger than taffeta, although thinner.
Fibrous non-impregnated electrical insulating materials
To produce such materials, organic fibers and artificially created components are used. Natural plant fibers of natural silk, flax, wood are converted into materials of organic origin (fiber, fabric, cardboard). The humidity of such insulators ranges from 6-10%.
Organic synthetic materials (nylon) contain moisture only from 3 to 5%; inorganic fibers (fiberglass) have the same moisture saturation. Inorganic materials are characterized by their inability to ignite when heated significantly. If materials are impregnated with enamels or varnishes, the flammability increases. The supply of electrical materials is carried out to an enterprise for the manufacture of electrical machines and devices.
Classification of electrical materials
Electrical materials are a set of conductor, electrical insulating, magnetic and semiconductor materials designed to operate in electric and magnetic fields. This also includes basic electrical products: insulators, capacitors, wires and some semiconductor elements. Electrical materials occupy one of the main places in modern electrical engineering. Everyone knows that the reliability of electrical machines, apparatus and electrical installations mainly depends on the quality and correct selection of appropriate electrical materials. Analysis of accidents of electrical machines and devices shows that most of them occur due to the failure of electrical insulation, consisting of electrical insulating materials.
Magnetic materials are no less important for electrical engineering. Energy losses and dimensions of electrical machines and transformers are determined by the properties of magnetic materials. Semiconductor materials, or semiconductors, occupy a fairly significant place in electrical engineering. As a result of the development and study of this group of materials, various new devices have been created that make it possible to successfully solve some problems in electrical engineering.
With a rational choice of electrical insulating, magnetic and other materials, it is possible to create electrical equipment that is reliable in operation with small dimensions and weight. But to realize these qualities, knowledge of the properties of all groups of electrical materials is required.
All bodies, depending on their electrical properties, can be classified as dielectrics, conductors or semiconductors. The difference between conductors, semiconductors and dielectrics can be most clearly shown using energy diagrams of the band theory of solids [3].
Energy levels.
Layout diagram.
Rice. 1.1 - normal energy level of the atom; 2—zone filled with electrons; 3—levels of the excited state of the atom; 4—free zone; 5 - prohibited zone.
A study of the emission spectra of various substances in the gaseous state, when the atoms are separated from each other at large distances, shows that the atoms of each substance are characterized by well-defined spectral lines. This indicates the presence of certain energy states (levels) for different atoms. Some of these levels are filled with electrons in the normal, unexcited state of the atom, while others can contain electrons only when the atom is subjected to external energy influence; at the same time he is excited. Trying to reach a stable state, the atom emits excess energy at the moment of transition of electrons from excited levels to levels at which its energy is minimal. This can be characterized by the energy diagram of an atom shown in Fig. 1.
When a gaseous substance condenses into a liquid and then forms a crystal lattice of a solid, all the electronic levels present in a given type of atom (both filled with electrons and unfilled) are slightly shifted due to the action of neighboring atoms on each other. Thus, from individual energy levels of solitary atoms in a solid a whole band is formed - a zone of energy levels.
Rice. 2. shows the difference in energy diagrams (at a temperature of 0 ° K) of metallic conductors, semiconductors and dielectrics. A dielectric is a body whose band gap is so large that electronic conductivity is not observed under normal conditions. Semiconductors will be substances with a narrower band gap, which can be overcome due to external energy influences. In metal conductors, the zone filled with electrons is closely adjacent to the zone of free energy levels or even overlaps it. As a result, the electrons in the metal are free, since they can move from the levels of the filled zone to the unoccupied levels of the free zone under the influence of weak electric field strengths applied to the conductor.
In the absence of free electrons in a semiconductor (T
= 0° K), an electrical potential difference applied to it will not cause a current. If energy sufficient to transfer electrons across the band gap is supplied from the outside, then, having become free, the electrons will be able to move under the influence of an electric field, creating electronic conductivity of the semiconductor.
Rice. 2. Energy difference between metal conductors and semiconductors and dielectrics
In the filled zone from which the electron left, an “electron hole” was formed, and therefore another “relay race” movement of electrons will begin in the semiconductor, filling the resulting hole, and under the influence of the electric field the hole will move in the direction of the field as an equivalent positive charge.
The process of transition of electrons to a free state is accompanied by the opposite phenomenon, i.e., the return of electrons to a normal state. As a result, equilibrium occurs in the substance, i.e., the number of electrons passing into the free zone becomes equal to the number of electrons returning back to the normal state.
With increasing temperature, the number of free electrons in a semiconductor increases, and with decreasing temperature to absolute zero, it decreases down to zero.
Thus, a substance that is a dielectric at some temperatures can acquire conductivity at other, higher temperatures; in this case, a qualitative change in the substance occurs.
The energy required to transfer an electron into a free state or to form a hole can be supplied not only by thermal motion, but also by other energy sources, for example, light energy absorbed by the material, the energy of the flow of electrons and nuclear particles, the energy of electric and magnetic fields, mechanical energy and etc.
An increase in the number of free electrons or holes in a substance under the influence of any type of energy contributes to an increase in electrical conductivity, an increase in current, and the appearance of electromotive forces.
Electrical properties are determined by the conditions of interaction between atoms of a substance and are not an indispensable feature of a given atom. For example, carbon in the form of diamond is a dielectric, but in the form of graphite it is highly conductive.
Impurities and associated lattice defects also play a large role in the electrical properties of solids.
Conductor materials
This group of materials includes metals and their alloys. Pure metals have low resistivity. The exception is mercury, which has a fairly high resistivity. The alloys also have high resistivity. Pure metals are used in the manufacture of winding and mounting wires, cables, etc. Conductor alloys in the form of wires and tapes are used in rheostats, potentiometers, additional resistances, etc.
In the subgroup of alloys with high resistivity, a group of heat-resistant conductor materials that are resistant to oxidation at high temperatures is distinguished. Heat-resistant, or heat-resistant, conductor alloys are used in electric heating devices and rheostats. In addition to low resistivity, pure metals have good ductility, i.e. they can be drawn into thin wire, into ribbons and rolled into foil less than 0.01 mm thick. Metal alloys have less ductility, but are more elastic and mechanically stable. A characteristic feature of all metallic conductor materials is their electronic conductivity. The resistivity of all metal conductors increases with increasing temperature, as well as as a result of mechanical processing, which causes permanent deformation in the metal.
Rolling or drawing is used when it is necessary to obtain conductor materials with increased mechanical strength, for example, in the manufacture of overhead line wires, trolley wires, etc. To return deformed metal conductors to their previous resistivity value, they are subjected to heat treatment - annealing without access to oxygen.
Solids, liquids, and, under appropriate conditions, gases can be used as conductors of electric current.
Metals are solid conductors. Metal conductor materials can be divided into high conductivity materials and high resistance materials. Metals with high conductivity are used for wires, cables, windings of transformers, electrical machines, etc. Metals and alloys of high resistance are used in electric heating devices, incandescent lamps, rheostats, standard resistances, etc.
Liquid conductors include molten metals and various electrolytes. As a rule, the melting point of metals is high, with the exception of mercury, for which it is about -39 ° C. Therefore, at normal temperatures, only mercury can be used as a liquid metal conductor. Other metals are liquid conductors at higher temperatures (for example, when melting metals with high frequency currents).
The mechanism of current flow through metals in solid and liquid states is determined by the movement of free electrons, as a result of which they are called conductors with electronic conductivity, or conductors of the first kind. Conductors of the second kind, or electrolytes, are solutions (mostly aqueous) of acids, alkalis and salts. The passage of current through these conductors is associated with the transfer of parts of the molecule (ions) along with electrical charges, as a result of which the composition of the electrolyte gradually changes, and electrolysis products are released on the electrodes.
Ionic crystals in the molten state are also conductors of the second kind. An example is salt quenching baths with electrical heating. All gases and vapors, including metal vapors, are not conductors at low electric field strengths. However, if the field strength exceeds a certain critical value that ensures the onset of impact and photoionization, then the gas can become a conductor with the presence of electronic and ionic conductivity. A highly ionized gas with an equal number of electrons and positive ions per unit volume represents a special conducting medium called plasma.
Metal conductors are the main type of conductor materials used in electrical engineering.
The classical electronic theory of metals represents a solid conductor in the form of a system consisting of nodes of a crystalline ionic lattice, inside which there is an electron gas of itinerant (free) electrons. In the itinerant state, one to two electrons are separated from each metal atom. When electrons collide with nodes of a crystal lattice, the energy accumulated during the acceleration of electrons in an electric field is transferred to the metal base of the conductor, as a result of which it heats up. It has been established as an experimental fact that the thermal conductivity of metals is proportional to their electrical conductivity.
When electrons are exchanged between heated and cold parts of a metal in the absence of an electric field, a transition of kinetic energy takes place from the heated parts of the conductor to the colder ones, i.e., a phenomenon called thermal conductivity. Since the mechanisms of electrical conductivity and thermal conductivity are determined by the density and movement of the electron gas, materials with high conductivity will also be good conductors of heat.
A number of experiments confirmed the hypothesis of electron gas in metals. These include the following:
1. When an electric current is passed for a long time through a circuit consisting of only metal conductors, the penetration of atoms of one metal into another is not observed.
2. When metals are heated to high temperatures, the speed of thermal movement of free electrons increases, and the fastest of them can fly out of the metal, overcoming the forces of the surface potential barrier.
3. At the moment of an unexpected stop of a rapidly moving conductor, the electron gas shifts according to the law of inertia in the direction of movement. The displacement of electrons leads to the appearance of a potential difference at the ends of the inhibited conductor, and the measuring device connected to them gives a deviation on the scale.
4. By studying the behavior of metal conductors in a magnetic field, it was established that due to the curvature of the electron trajectory in a metal plate placed in a transverse magnetic field, a transverse e. appears. d.s. and the electrical resistance of the conductor changes.
The main characteristics of conductor materials include:
1) specific conductivity or its reciprocal value - electrical resistivity;
2) temperature coefficient of resistivity;
3) thermal conductivity;
4) contact potential difference and thermoelectromotive force (thermo - emf s);
5) tensile strength and elongation at break.
The most widely used high conductivity materials include copper and aluminum.
The advantages of copper, which ensure its widespread use as a conductor material, are as follows:
1) low resistivity (of all metals, only silver has a slightly lower resistivity than copper);
2) sufficiently high mechanical strength;
3) resistance to corrosion is satisfactory in most cases of application (copper oxidizes in air, even in conditions of high humidity, much more slowly than, for example, iron); intense oxidation of copper occurs only at elevated temperatures;
4) good workability - copper is rolled into sheets, strips and drawn into wire, the thickness of which can be increased to thousandths of a millimeter;
5) relative ease of soldering and welding.
The second most important conductor material, after copper, is aluminum. This is a silver-white metal, the most important representative of the so-called light metals; aluminum is approximately 3.5 times lighter than copper. The thermal coefficient of linear expansion, specific heat capacity and heat of fusion of aluminum are greater than those of copper.
Due to the high values of specific heat capacity and heat of fusion, heating aluminum to the melting point and transferring it to a molten state requires more heat than heating and melting the same amount of copper, although the melting point of aluminum is lower than copper.
Aluminum has lower properties compared to copper - both mechanical and electrical. With the same cross-section and length, the electrical resistance of an aluminum wire is 0.028: 0.0172 = 1.63 times greater than that of a copper wire. Therefore, in order to obtain an aluminum wire with the same electrical resistance as copper, you need to take its cross-section 1.63 times larger than the diameter of the copper wire. Aluminum wire, although thicker than copper, is approximately two times lighter.
This leads to a simple economic rule: for the manufacture of wires of the same conductivity for a given length (i.e., other things being equal, with the same losses of transmitted electrical energy), aluminum is more profitable than copper if a ton of aluminum is more expensive than a ton of copper no more than twice.
Currently, in our country, based on economic considerations, aluminum has not only, as a rule, replaced copper for overhead transmission lines, but is also beginning to be introduced into the production of insulated cable products.
Semiconductor materials
Semiconductors include a large number of materials that differ from each other in internal structure, chemical composition and electrical properties. According to their chemical composition, crystalline semiconductor materials are divided into 4 groups:
1. materials consisting of atoms of one element: germanium, silicon, selenium, phosphorus, boron, indium, gallium, etc.;
2. materials consisting of metal oxides: cuprous oxide, zinc oxide, cadmium oxide, titanium dioxide, etc.;
3. materials based on compounds of atoms of the third and fifth groups of the Mendeleev system of elements, denoted by a general formula and called antimonides. This group includes compounds of antimony with indium, with gallium, etc., compounds of atoms of the second and sixth groups, as well as compounds of atoms of the fourth group;
4. semiconductor materials of organic origin, for example polycyclic aromatic compounds: anthracene, naphthalene, etc.
According to the crystal structure, semiconductor materials are divided into 2 groups: monocrystalline and polycrystalline semiconductors. The first group includes materials obtained in the form of large single crystals (single crystals). Among them are germanium and silicon, from which plates are cut for rectifiers and other semiconductor devices.
The second group of materials are semiconductors, consisting of many small crystals soldered to each other. Polycrystalline semiconductors are: selenium, silicon carbide, etc.
In terms of volumetric resistivity, semiconductors occupy an intermediate position between conductors and dielectrics. Some of them sharply reduce electrical resistance when exposed to high voltage. This phenomenon has found application in valve-type arresters to protect power lines. Other semiconductors dramatically decrease their resistance when exposed to light. This is used in photocells and photoresistors. A common property for semiconductors is that they have electron and hole conductivity.
A large group of substances with electronic electrical conductivity, the resistivity of which at normal temperature lies between the resistivities of conductors and dielectrics, can be classified as semiconductors.
The electrical conductivity of semiconductors depends to a large extent on external energy influences, as well as on various impurities, sometimes present in minute quantities in the body of the semiconductor itself. The controllability of the electrical conductivity of semiconductors by temperature, light, electric field, and mechanical forces is, accordingly, the basis of the operating principle of thermistors (thermistors), photoresistors, nonlinear resistors (varistors), strain gauges, etc.
The presence of two types of electrical conductivity in semiconductors - “electronic ” (n) *
and “electron-hole”
(p)
allows you to obtain semiconductor products with
a p-n
junction.
When p— n —
At the transition, a blocking layer appears, which determines the rectifying effect for alternating current. The presence of two or more mutually connected transitions makes it possible to obtain controlled systems - transistors.
On using the capabilities of p
-n
-
junctions are the basis for the most important applications of semiconductors in electrical engineering. This includes various types of both high- and low-power rectifiers, amplifiers and generators. Semiconductor systems can be successfully used to convert various types of energy into electrical energy with conversion efficiency values that make them comparable to, and sometimes superior to, existing other types of converters. Examples of semiconductor converters can be “solar batteries” with an efficiency of about 11% and thermoelectric generators.
With the help of semiconductors, it is possible to achieve cooling of several tens of degrees. In recent years, recombination luminescence at low DC voltage of electron-hole junctions has acquired particular importance for the creation of signal light sources. In addition to the above main applications of semiconductors, they can serve as heating elements (silite rods), they can be used to excite a cathode spot in ignitron rectifiers (ignitron igniters), measure the magnetic field strength (Hall sensors), they can be indicators of radioactive radiation, etc. Semiconductor materials used in practice can be divided into simple semiconductors (elements), semiconductor chemical compounds and semiconductor complexes (for example, ceramic semiconductors). Glassy and liquid semiconductors are also currently being studied.
There are about ten simple semiconductors. For modern technology, germanium, silicon and selenium are of particular importance.
Semiconductor chemical compounds are compounds of elements of various groups of the periodic table.
Multiphase semiconductor materials include materials with a semiconducting or conducting phase made of silicon carbide, graphite, etc., bonded with a ceramic or other binder. The most common of them are tirite, silit, etc.
Devices made from semiconductor materials have a number of advantages; These include:
1) long service life;
2) small dimensions and weight;
3) simplicity and reliability of design, high mechanical strength (not afraid of shaking and shock);
4) semiconductor devices that replace vacuum tubes do not have filament circuits, consume little power and have low inertia;
5) when mastered in mass production, they are economically feasible.
Domestic science and technology of semiconductors developed in its own way, enriching world science with its achievements and successes and at the same time, using everything progressive that foreign science and technology provided, through the creative development of the practical results of foreign work.
Magnetic materials
Magnetism is a special manifestation of the movement of electrical charges within atoms and molecules, which manifests itself in the fact that some bodies are able to attract and hold particles of iron, nickel and other metals. These bodies are called magnetic.
A magnetic field arises around any magnetized body, which is a material medium in which the action of magnetic forces is detected.
When a body is introduced into a magnetic field, it is penetrated by magnetic lines, which influence the field in a certain way. At the same time, different materials have different effects on the magnetic field. In magnetized bodies, a magnetic field is created by the movement of electrons rotating around the nucleus of an atom and around its own axis. The orbits and axes of rotation of electrons in atoms can be in different positions relative to each other, so that magnetic fields excited by moving electrons are in different positions. Depending on the relative position of the magnetic fields, they can be added or subtracted. In the first case, the atom will have a magnetic field or magnetic moment, but in the second it will not. Materials whose atoms do not have a magnetic moment and which cannot be magnetized are called diamagnetic. These include the vast majority of substances found in nature and some metals (copper, lead, zinc, silver and others). Materials whose atoms have a certain magnetic moment and can be magnetized are called paramagnetic. These include aluminum, tin, manganese, etc. The exception is ferromagnetic materials, the atoms of which have a large magnetic moment and are easily magnetized. Such materials include iron, steel, cast iron, nickel, cobalt, gadolinium and their alloys.
The property of electric current to create a magnetic field is widely used in practice.
An iron or steel rod placed inside a solenoid becomes magnetic when current is passed through the solenoid. A rod of hard magnetic steel, due to the large coercive force inherent in this material, largely retains its magnetic properties even after the current disappears.
Electronics and communications devices often use polarized electromagnets, in which either the core, the armature, or both are magnets.
An unpolarized electromagnet attracts its armature regardless of the direction of the current sent to its winding. The operation of a polarized electromagnet depends on the direction of the current in its winding. So, for example, in a directly polarized electromagnet, a current in one direction strengthens the magnetic field of its core, and weakens the other.
Electromagnets are widely used in lifting and braking devices, for securing steel workpieces in machine tools, in electric machines, relays and other devices.
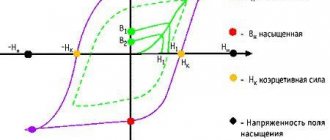
The quantities by which the magnetic properties of materials are assessed are called magnetic characteristics. These include: absolute magnetic permeability, relative magnetic permeability, temperature coefficient of magnetic permeability, maximum magnetic field energy, etc. All magnetic materials are divided into two main groups: soft magnetic and hard magnetic.
Magnetically soft materials are characterized by low hysteresis losses (magnetic hysteresis is a lag between the magnetization of a body and the external magnetizing field). They have relatively large magnetic permeability values, low coercive force and relatively high saturation induction. These materials are used for the manufacture of magnetic cores of transformers, electrical machines and devices, magnetic screens and other devices where magnetization with low energy losses is required.
Hard magnetic materials are characterized by large hysteresis losses, i.e., they have high coercive force and high residual induction. These materials, being magnetized, can retain the resulting magnetic energy for a long time, i.e., they become sources of a constant magnetic field. Hard magnetic materials are used to make permanent magnets.
According to their basis, magnetic materials are divided into metallic, nonmetallic and magnetodielectrics. Metallic magnetically soft materials include: pure (electrolytic) iron, sheet electrical steel, iron-Armco, permalloy (iron-nickel alloys), etc. Metallic magnetically hard materials include: alloy steels, special alloys based on iron and aluminum and nickel and alloying components (cobalt, silicon, etc.). Non-metallic magnetic materials include ferrites. These are materials obtained from a powdery mixture of oxides of certain metals and iron oxide. Pressed ferrite products (cores, rings, etc.) are fired at a temperature of 1300-1500° C. Ferrites are either magnetically soft or magnetically hard.
Magnetodielectrics are composite materials consisting of 70-80% powdered magnetic material and 30-20% organic high-polymer dielectric. Ferrites and magnetodielectrics differ from metal magnetic materials in having higher volume resistivity values, which sharply reduces eddy current losses.
Leteroid
Thin fiber is produced in sheets and rolled into a roll for transportation. It is used as a material for the manufacture of insulation gaskets, shaped dielectrics, and washers. Asbestos-impregnated paper and asbestos cardboard are made from chrysolite asbestos by splitting it into fibers. Asbestos is resistant to alkaline environments, but is destroyed in acidic environments.
In conclusion, it should be noted that with the use of modern materials for insulating electrical appliances, their service life has significantly increased. Materials with selected characteristics are used for installation housings, which makes it possible to produce new functional equipment with improved performance.
Solid dielectrics
Solid electrical insulating materials are the widest class of dielectrics that are used in various fields. They have different chemical properties, and the dielectric constant ranges from 1 to 50,000.
Solid dielectrics are divided into non-polar, polar and ferroelectrics. Their main differences are in the polarization mechanisms. This class of insulation has properties such as chemical resistance, tracking resistance, and dendrite resistance. Chemical resistance is expressed in the ability to withstand the influence of various aggressive environments (acid, alkali, etc.). Tregging resistance determines the ability to withstand the effects of an electric arc, and dendrite resistance determines the formation of dendrites.
Solid dielectrics are used in various fields of energy. For example, ceramic electrical insulating materials are most often used as line and bushing insulators in substations. Paper, polymers, and fiberglass are used as insulation for electrical devices. For machines and devices, varnishes, cardboard, and compound are most often used.
For use in various operating conditions, insulation is given some special properties by combining different materials: heat resistance, moisture resistance, radiation resistance and frost resistance. Heat-resistant insulators can withstand temperatures up to 700 °C; these include glass and glass-based materials, organosilites and some polymers. The moisture-resistant and tropical-resistant material is fluoroplastic, which is non-hygroscopic and hydrophobic.
Radiation resistant insulation is used in devices containing atomic elements. This includes inorganic films, some types of polymers, fiberglass and mica-based materials. Frost-resistant insulation is considered to be one that does not lose its properties at temperatures down to -90 °C. Special requirements are placed on insulation intended for devices operating in space or vacuum conditions. For these purposes, vacuum-dense materials are used, which include special ceramics.