During the operation of nickel-cadmium batteries, a feature called the “memory effect” was identified. Later, this feature was also revealed when using batteries of other types of chemistry. Its essence lies in the reversible loss of capacity that occurs under certain recharging modes, including when recharging partially discharged cells.
The battery seems to remember that the last time its capacity was not fully used, and in subsequent times it releases less energy, i.e. its original capacity decreases. This phenomenon intensifies when batteries are systematically recharged from a partially charged state, especially by 50% or more. Lithium-ion batteries have a memory effect, but it is not pronounced, which distinguishes them favorably from nickel-cadmium and nickel-metal hydride cells.
Definition of the “memory effect” of a battery
The “memory effect” of a battery is a process of significant loss of battery capacity due to violations of charging conditions, when the battery begins to be charged before the previous charge is lost.
In this case, conditions are created so that the power source seems to fix the capacity level until the moment when it begins to be recharged. In further operation, the battery will deliver current strictly up to this value.
The battery has no brain and, accordingly, no memory, and the described effect is explained by an increase in the number of crystals of the active substance.
This deviation becomes especially pronounced when rechargeable batteries are recharged before they have fully delivered the current they still have.
If this cycle is repeated over and over again, the battery loses most of its capacity. In addition, there is a risk that the product will completely lose its functionality.
Signs that a battery is showing signs of this phenomenon are not difficult - they are similar between models from different manufacturers. To protect yourself from changing the battery more often than necessary, you need to pay attention to the operating mode of the battery and its deviations from the standard.
The number of battery charge cycles is finite, but it is possible to revive even significantly damaged batteries through specific training.
Advantages of Li-ion technology
Lithium-ion batteries are used in most modern devices for good reason. Since their creation in 1991, they have made a great name for themselves and have significantly pushed aside their competitors. And although the search for the ideal battery continues to this day, lithium-ion batteries still remain the undisputed leaders.
Unlike Ni-Mh batteries, Li-ion models have:
- service life of more than 1000 cycles – until the initial capacity reserve is reduced by 20%;
- higher energy intensity – up to 230 Wh/dm³;
- minimum self-discharge – less than 5% per month, up to 20% per year;
- absolutely unexpressed memory effect;
- comfortable operating conditions - with the ability to recharge at any time, regardless of the level of residual charge;
- quick charge replenishment;
- nominal voltage 3.6–3.7 V per element;
- operating temperature range from −20 to +60 °C.
Li-ion batteries also have disadvantages: reduced capacity in the cold, fear of overcharging and deep discharge, risk of fire if depressurized or short-circuited. To eliminate these shortcomings, batteries and batteries are equipped with control and protection boards.
The BMS board clearly monitors operating parameters and prevents dangerous conditions. In particular, it disconnects dead batteries from the load, controls the level and uniformity of charge on all battery cells and stops the charging process when the voltage reaches its maximum. If you have a working protection board and follow simple safety rules, Li-ion batteries are absolutely safe to use.
Variants of manifestation of the “memory effect” in the battery
Awareness of the battery type is an important factor when dealing with the memory effect of a product. Crystal growth is a phenomenon to which batteries with different electrolyte and electrode chemistries are susceptible differently:
1. Ni-Mh. If the battery contains nickel, it can be said with a high probability that it will be prone to the “memory effect” (Ni-Mh batteries as well).
Once you break the charging mode, the next operating cycle will be much shorter. And if the device in which the battery is installed is used constantly, the manifestation of the “memory effect” will be reflected in a significant reduction in its operating time.
2. Ni-Cd. This type of battery is the most sensitive in terms of the “memory effect”. A special feature is that a decrease in operating time as a result of a reduction in capacity can occur even after short-term operation. The effect is most noticeable in inexpensive models.
What is the solution
There are two ways out of this situation:
- use the REFRESH function;
- restore your memory on your own.
If we talk about the first function, then it is supported in many chargers. Its essence lies in the fact that it allows you to recharge the battery to the bottom aisle before charging. As a result, only the correct memory is retained and the capacity will last much longer.
The second way is self-treatment. The bottom line is that you need to completely discharge and charge your capacity to maximum several times. As a rule, such manipulations will help completely correct the problem. However, if the container is already worn out, then nothing will help it.
Remember! This method cannot be applied to modern batteries under any circumstances. They should not be fully charged or discharged!
Ways to prevent the development of the “memory effect”
It is extremely easy to prevent the “memory effect” in batteries that are predisposed to it. You just need to stay set to charge the battery before it is completely depleted.
If this is not possible, then for preventive purposes it is necessary to periodically carry out a cycle to completely consume the current, followed by full recharging using the recommendations of the battery manufacturer.
In Ni-Cd and nickel-metal hydride batteries, the approach to reducing the risk of developing the memory effect is different.
You must set the required capacity level. To do this, you need a new battery all the way, using the amperage recommended by the product manufacturer.
Next, you need to completely discharge the battery using a low-power device.
Such a cycle will make it possible to set the maximum possible performance characteristics of the product at the start of operation, as well as remove the primary formation of crystals on the internal contacts of the battery.
When it meets
Let's pay attention! Modern batteries do not have a memory effect. Accordingly, there is no point in constantly charging or discharging them. They work at full capacity and show stable operation, which is why such batteries for phones began to be used.
The following batteries are currently susceptible to memory effect:
- Metal-Hydride NI-MH.
- Nickel Kadim NI-Cd.
Such batteries are not so easy to find now, but they are still found on our territory.
Methods for restoring battery capacity
Restoring the capacity of nickel-containing batteries is almost complete. The procedure must be performed in a specific order:
- discharge the battery through a low-power device to a voltage at the contacts of 0.8-1.0 V (a multimeter is used for measurement);
- fully charge the battery using a charger;
- repeat the steps of the previous steps 2-5 times.
If the battery is placed on recharge when the available electrical current has not been completely used up for a significant period of time, it is likely that a higher power charger will be required to restore capacity.
The procedure described above can also be carried out as a preventive measure. It is applicable in several cases:
- the “memory effect” is not very obvious;
- the device is not used for a long time or is not charging;
- The battery used is of the Ni-Mh or Ni-Cd type.
Which devices are most susceptible to the problem?
The memory effect is especially strong in portable devices that can be used for long periods of time. For example, screwdrivers used in non-electrified objects are charged to full capacity, even if the electricity supply is not used up.
This is due, first of all, to the fact that during the work process it will not be possible to install the device for recharging. A similar problem occurs if the wireless device is used periodically.
Workers, when there are breaks in using the device, connect it to the network via an adapter, which leads to a very rapid decrease in the efficiency of the power supply.
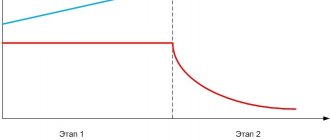
Ultra-fast charge: charge current 0.5...1 C
At this current, automatic detection of the end of charging is possible. You can charge in automatic mode if you have an automatic charger, but if not, you need to calculate the time using the above formula.
The batteries may become very hot, this is normal. If the heating is above 55°C, you need to turn off the charge and wait for it to cool down. The approximate temperature can be assessed by touching the batteries for a long time - if there is a burning sensation and it is impossible to maintain contact for a long time, then the temperature is 55...60°C.
Remember that not all batteries support ultra-fast charging.
Temperature
Standard charge: 0…45ºС
Fast charge: 10…45ºС
Discharge: 20…65ºС
Overheating often occurs when batteries are charged with high current. The temperature when charging with current is more than 0.5 C, where C is the capacity, and can reach 65 ° C, therefore, when using fast chargers, accelerated aging of batteries is inevitable.
Some chargers have a cooler or an overheating protection system - they stop the charging process when a certain temperature threshold is exceeded.
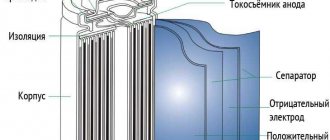
Design
The battery includes two electrodes charged with opposite charges, between which there is a separator, that is, a delimiter. Each cell contains an electrolyte and is hermetically sealed in a housing, usually made of metal or plastic.
An electrode with a positive charge contains nickel hydroxide oxide. The negative one is made of cadmium. The electrolyte is highly active alkali KOH - odorless and non-explosive.
When making electrodes, the metal is foil-coated - this makes it possible to increase the interaction area. The separator is a material that is resistant to alkali. Since the battery case is sealed, it does not lose electrolyte during operation.
The current collector contacts are located on top of the battery. The housing has a hole for refilling
Battery design.
alkalis. Excess gases formed during battery charging are released through it. Special plates serve as electrodes, and the separator separating them does not limit the flow of electrolyte.
In terms of design, the Ni-Cd battery power supply can be:
- prismatic. Electrodes are plates stacked on top of each other through a separator;
- cylindrical. The electrodes have the form of a tape rolled into a roll. A separator is also present - between the anode and cathode.
Advantages and disadvantages
A significant increase in the energy parameters of nickel-metal hydride batteries is not their only advantage over cadmium batteries. Having abandoned the use of cadmium, manufacturers began to use a more environmentally friendly metal. Disposal issues are much easier to resolve.
Due to these advantages and the fact that the metal used in manufacturing is nickel, the production of Ni-MH devices has increased sharply when compared with nickel-cadmium batteries. They are also convenient because in order to reduce the discharge voltage during long-term recharges, a full discharge (up to 1 volt) must be carried out once every 20-30 days.
A little about the disadvantages:
- Manufacturers have limited Ni-MH batteries to ten cells , because with increasing charge-discharge cycles and service life there is a danger of overheating and polarity reversal.
- These batteries operate in a narrower temperature range than nickel-cadmium batteries . Already at -10 and +40°C they lose their performance.
- When charging Ni-MH batteries, they generate a lot of heat , so they need fuses or temperature relays.
- Increased self-charge , the presence of which is due to the reaction of the nickel oxide electrode with hydrogen from the electrolyte.
Degradation of Ni-MH batteries is determined by a decrease in the sorption capacity of the negative electrode during cycling. During the discharge-charge cycle, a change in the volume of the crystal lattice occurs, which contributes to the formation of rust and cracks during the reaction with the electrolyte. Corrosion occurs when the battery absorbs hydrogen and oxygen. This leads to a decrease in the amount of electrolyte and an increase in internal resistance.
It must be taken into account that the characteristics of batteries depend on the processing technology of the negative electrode alloy, its structure and composition. The metal for alloys also matters. All this forces manufacturers to very carefully choose alloy suppliers, and consumers - the manufacturer.
How are these devices used?
Nickel-metal hydride devices are widely used to power various types of electronics that operate in autonomous mode. They are usually made in the form of AAA or AA batteries. Other versions are also available. For example, industrial batteries. The scope of use of Ni-MH batteries is slightly wider than that of nickel-cadmium batteries, because they do not contain toxic materials.
Currently, nickel-metal hydride batteries sold on the domestic market are divided into 2 groups based on capacity - 1500-3000 mAh and 300-1000 mAh:
- The first is used in devices that have increased energy consumption in a short time. These are all kinds of players, radio-controlled models, cameras, video cameras. In general, devices that quickly consume energy.
- The second is used for energy consumption that begins after a certain time interval. These are toys, flashlights, walkie-talkies. Battery-powered devices operate on batteries that consume electricity moderately and remain offline for a long time.
Operating rules
When using such batteries, you need to understand that during operation changes occur in them, leading to a decrease in operational parameters, and subsequently to failure:
- the area and mass of the electrodes are reduced;
- the volume and composition of the electrolyte also undergoes changes;
- the separator is deformed;
- water and oxygen are consumed;
- Dendrites appear on the plates, causing current leakage.
There are operating rules that, if followed, can actually extend the “life” of the battery. What factors can affect battery performance:
- if you charge a battery that is not fully charged, its total capacity will decrease - due to crystal formation, the area of the substance decreases;
- Overcharging is not allowed - this not only increases gas formation, but also leads to loss of water in the electrolyte and destruction of the electrodes and separator;
- due to undercharging, the battery will quickly deplete;
- Regardless of the fact that a Ni-Cd type battery can be used at low temperatures, it will not last long in this scenario - due to frost, the volume of the electrolyte will decrease, its composition will change, and as a result, the capacity will drop.
A fast charge with a high current should also not be practiced - the pressure in the battery case will increase and the cadmium cathode will begin to degrade. This will lead to deformation of the housing, loss of density, increase in internal resistance and decrease in operating voltage.
Batteries are produced with a pressure relief valve, which prevents deformation, but if the chemical composition changes, nothing can be corrected.
How to charge the battery
To recharge, a current of 10% of the capacity parameter is required (100 mA per 1000 mAh) for 14 - 16 hours. Recommended current is 20% of capacity. If you do not adhere to these values, the pressure inside will begin to increase and oxygen will be released, which will lead to irreversible consequences.
The charger circuit involves the use of automatic and reverse charging.
Charging efficiency at different charge rates.
Storage and disposal
Battery discharge characteristics.
Optimal storage conditions for a discharged battery: dry place and low temperature. Self-discharge will be less if the ambient temperature is sub-zero. If we are talking about high-quality models, they can remain idle for about 5 years, and their technical characteristics will not change. To start using the battery, you only need to charge it.
Ni-Cd power supplies should not be thrown into the trash. One battery contains enough harmful substances to pollute 20 m2 of territory. Recycling involves handing over the battery to recycling points.