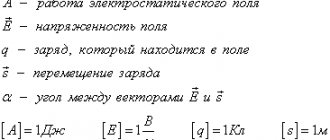Just like the gravitational mass of a body in Newtonian mechanics, charge in electrodynamics refers to fundamental, basic concepts.
Electric charge
This is a physical quantity that means the property of some particles or bodies to be involved in electromagnetic interactions. In physics, electric charge is usually denoted q, less often Q.
The following conclusions follow from the established experimental facts:
- in nature there are two types of electrical charges , conventionally “positive” (+) and “negative” (-);
- charges are transferred from one body to another (for example, in the case of direct contact of two objects). Therefore, electric charge , unlike body mass, is not a constant characteristic of a particular body. The same body under different conditions can have different charges.
- Like charges repel, opposite charges attract . That is, “+” repels “+”, “-” repels “-”. But “+” attracts “-” and vice versa.
Pendant.
A coulomb is a unit of measurement of electrical charge (amount of electricity) as well as the flux of electrical induction (flux of electrical displacement) in the International System of Units (SI). It has a Russian designation - Kl and an international designation - C.
Pendant as a unit of measurement
Application of the pendant
Representation of a pendant in other units of measurement - formulas
Multiples and submultiples of coulomb units
Interesting examples
Other units of measurement
Measuring the amount of charge
The standard method for detecting and measuring charge is a device - an electrometer . It consists of a metal rod and an arrow rotating around a horizontal axis. The rod and pointer are isolated from the metal body of the device. When a charged body touches the rod of the device, electric charges of the same sign flow along the rod and the arrow. Electrostatic repulsion forces rotate the arrow through a certain angle. By the magnitude of the angle one can judge the charge that was transferred to the electrometer rod.
In practice, the concept of a point charge is often used. A point charge is a charged body whose dimensions can be neglected.
Pendant as a unit of measurement:
A coulomb is a unit of measurement of electrical charge (amount of electricity) as well as flux of electrical induction (flux of electrical displacement) in the International System of Units (SI), named after the French physicist and engineer Charles Coulomb.
The pendant as a unit of measurement has a Russian designation - Kl and an international designation - S.
1 coulomb is defined as the amount of charge passing through a conductor at a current of 1 ampere in 1 second.
Cl = A · s.
1 C = 1 A s = 1/3600 ampere hour.
The charge of one pendant is very large. If two charge carriers (q1 = q2 = 1 C) were placed in a vacuum at a distance of 1 m, then they would interact with a force of 9⋅109 N, that is, with the force with which the Earth’s gravity attracts an object weighing about 1 million tons.
Electric charge (amount of electricity) is a physical scalar quantity. Electric charge carriers are electrically charged elementary particles (electron, positron, proton, etc.). The smallest particle by mass that is stable in a free state and has one negative elementary electric charge is the electron. The electric charge of an electron is indivisible and equal to -1.6021766208(98)⋅10−19 C. The charge of a proton is also equal to the charge of an electron, but with the opposite sign (+ sign) and is equal to +1.6021766208(98)⋅10−19 C.
Thus, the elementary electric charge (to within a sign equal to the charge of an electron or proton) is the above value +/- 1.602176 6208(98)⋅10−19 C. Accordingly, the electric charge of 6.24151⋅1018 electrons is equal to -1 C, and the electric charge of 6.24151⋅1018 protons is equal to +1 C. In this case, the mass of the electron is 9.10938356(11)⋅10−31 kg, and that of the proton is 1.672 621 923 69(51)⋅10−27 kg.
The smallest antiparticle stable in a free state with a positive elementary charge is the positron, which has the same electric charge as the electron, but with a + sign. The electric charge of a positron is +1.6021766208(98)⋅10−19 C. The positron mass is 9.10938356(11)⋅10−31.
The coulomb was introduced into the International System of Units by the decision of the XI General Conference on Weights and Measures in 1960, simultaneously with the adoption of the SI system as a whole. In accordance with the SI rules regarding derived units named after scientists, the name of the unit “coulomb” is written with a lowercase letter, and its designation with a capital letter (Cl). This spelling of the designation is also preserved in the designations of derived units formed using a pendant.
Coulomb's law
One of the basic laws of nature is the experimentally established law of conservation of charge (better known as “Coulomb’s Law”).
In a closed system, the algebraic sum of charges is conserved:
q 1 + q 2 + q 3 + … + qn = const q_1 + q_2 + q_3 + … + q_n = const q1+q2+q3+…+qn=const
This law also means that processes of the appearance or disappearance of charges of only one sign cannot occur in an isolated system. That is, charges are born and die in pairs (“+” with “-”).
In modern science, charge carriers are elementary particles. All bodies in the Universe are made of atoms. But atoms, in turn, consist of such elementary particles. Positively charged protons, negative electrons and particles without a charge - neutrons. Protons and neutrons are part of the nucleus of an atom (therefore it is positively charged), and electrons are part of the shell (negatively charged). In a neutral atom, the charge of the nucleus is equal to the charge of all the electrons in the shell. The charge of a proton and an electron are equal in value.
It has been experimentally shown that charge can be transferred from one body to another only in whole portions or discretely:
q = ±ne ( n = 0, 1, 2, … ) , q = ± ne (n = 0, 1, 2, …), q=±ne(n=0,1,2,…),
eee – electron charge.
Multiples and submultiples of the coulomb:
Multiples and submultiples are formed using standard SI prefixes.
| Multiples | Dolnye | ||||||
| magnitude | Name | designation | magnitude | Name | designation | ||
| 101 Kl | deca pendant | yesCl | daC | 10−1 Cl | decicoulone | dCL | dC |
| 102 Kl | hectocoulomb | gC | hC | 10−2 Cl | centicoulomb | sKl | cC |
| 103 Kl | kilocoulomb | kCl | kC | 10−3 Cl | milliculomb | mC | mC |
| 106 Kl | mega pendant | MCL | M.C. | 10−6 Cl | microcoulomb | µC | µC |
| 109 Kl | gigakulon | GKl | G.C. | 10−9 Cl | nanocoulomb | nCl | nC |
| 1012 Cl | terakulon | TKl | TC | 10−12 Cl | picoculomb | pCL | pC |
| 1015 Kl | petacoulomb | Pcl | PC | 10−15 Kl | femtocoulomb | fkl | fC |
| 1018 Kl | exaculon | ECL | E.C. | 10−18 Cl | attocoulomb | aKl | aC |
| 1021 Kl | zettacoulomb | ZKl | ZC | 10−21 Cl | zeptoculone | zkl | zC |
| 1024 Kl | iottakulon | ICL | YC | 10−24 Cl | ioctocoulomb | iKl | yC |
History of discovery
Many physicists have carried out experiments with charged particles:
- G. V. Richman;
- professor of physics F. Epinus;
- D. Bernoulli;
- Priestley;
- John Robison and many others.
All these scientists came very close to discovering the law, but none of them managed to mathematically substantiate their guesses. Undoubtedly, they observed the interaction of charged balls, but establishing a pattern in this process was not easy.
Coulomb carried out careful measurements of the interaction forces. For this purpose, he even designed a unique device - a torsion balance (see Fig. 2).
Rice. 2. Torsion scales
The scales invented by Coulomb had extremely high sensitivity. The device responded to forces of the order of 10-9 N. The yoke of the scales, under the influence of this tiny force, rotated by 1º. The experimenter could measure the angle of rotation, and therefore the applied force, using an accurate scale.
Thanks to the scientist’s brilliant guess, the idea was that when charged and uncharged balls came into contact, the electric charge was divided equally between them. The torsion scales immediately responded to this, the beam of which was rotated to a certain angle. By grounding the stationary ball, Coulomb could neutralize the resulting charge on it.
Thus, the scientist was able to reduce the initial charge of the moving ball a multiple of times. Measuring the angle of deflection after each division of the charge, Coulomb saw a pattern in the action of the repulsive force, which helped him formulate his famous law.
Application in practice
All modern electrical engineering is built on the principles of interaction of Coulomb forces. Thanks to Clone's discovery of this fundamental law, an entire science has developed that studies electromagnetic interactions. The concept of the term electric field is also based on knowledge of Coulomb forces. It has been proven that the electric field is inextricably linked with the charges of elementary particles.
Thunderclouds are nothing more than a collection of electrical charges. They attract induced earth charges towards themselves, resulting in lightning. This discovery made it possible to create effective lightning rods to protect buildings and electrical structures.
Many inventions have appeared based on electrostatics:
- capacitor;
- various dielectrics;
- antistatic materials to protect sensitive electronic parts;
- protective clothing for electronics industry workers and much more.
The operation of charged particle accelerators, in particular, the operation of the Large Hadron Collider (see Fig. 4), is based on Coulomb’s law.
Rice. 4. Large Hadron Collider
Acceleration of charged particles to near-light speeds occurs under the influence of an electromagnetic field created by coils located along the path. Elementary particles disintegrate from the collision, traces of which are recorded by electronic devices. Based on these photographs, applying Coulomb's law, scientists draw conclusions about the structure of the elementary building blocks of matter.
References:
- Sivukhin D.V. General course in physics. - M.: Fizmatlit; Publishing house MIPT, 2004.
- Landau L. D., Lifshits E. M. Theoretical physics: Textbook. manual: For universities.
- Landsberg G.S. Elementary physics textbook. Volume II. Electricity and magnetism.
Notes
- Dengub V. M., Smirnov V. G.
Units of quantities. Dictionary-reference book. - M.: Standards Publishing House, 1990. - P. 67. - 240 p. — ISBN 5-7050-0118-5. Derived units Becquerel · Watt · Weber · Volt · Henry · Hertz · Celsius · Gray · Joule · Sievert · Cathal · Coulomb · Lux · Lumen · Newton · Newton-meter · · Pascal · Radian · Siemens · Steradian · Tesla · Farad Accepted for use with SI Angstrom · Astronomical unit · Hectare · Degree of arc (Minute of arc, Second of arc) · Dalton (Atomic unit of mass) · Decibel · Liter · Naper · Day (Hour, Minute) · Ton · Electronvolt Atomic system of units · Natural system of units see also SI prefixes · System of physical quantities · Unit conversions · New definitions of SI · History of the metric system Book:SI · Category:SI This is a preliminary article about units of measurement. You can help the project by adding to it.
Formulation
Coulomb studied the interaction between balls of negligible size compared to the distances between them. In physics, such charged bodies are called point bodies. In other words, such charged bodies fall under the definition of point charges if their dimensions, under the conditions of a particular experiment, can be neglected.
For point charges, the following statement is true: The interaction forces between them are directed along a line passing through the centers of the charged bodies. The absolute magnitude of each force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them (see Fig. 3). This dependence can be expressed by the formula: |F1|=|F2|=(ke*q1*q2) / r2
Rice. 3. Interaction of point charges
It remains to add that the force vectors are directed towards each other for unlike charges, and in the opposite direction in the case of like charges. That is, there is electrical attraction between like charges, and repulsion between like charges.
Thus, Coulomb's law describes the interaction between two electric charges, which underlies all electromagnetic interactions.
In order for the above law to apply, the following conditions must be met:
- compliance with point charges;
- immobility of charged bodies;
- the law expresses the relationships between charges in a vacuum.
Limits of application
The pattern described above, under certain conditions, is applicable to describe the processes of quantum mechanics. True, Coulomb's law is formulated without the concept of force. Instead of force, the concept of potential energy of the Coulomb interaction is used. The pattern was obtained by generalizing experimental data.
It should be noted that at ultra-short distances (during interactions of elementary particles) of the order of 10 - 18 m, electroweak effects appear. In these cases, Coulomb's law, strictly speaking, is no longer observed. The formula can be applied subject to amendments.
Violation of Coulomb's law is also observed in strong electromagnetic fields (about 1018 V/m), for example, near magnetars (a type of electron star). In such an environment, the Coulomb potential decreases not in inverse proportion, but exponentially.
Coulomb forces are subject to Newton's third law: F1 = – F2 . They are used to describe the laws of gravity. In this case, the formula takes the form: F = ( m1* m2 ) / r2 , where m1 and m2 are the masses of interacting bodies, and r is the distance between them.
Coulomb's law became the first open quantitative fundamental law substantiated mathematically. Its importance in the study of electromagnetic phenomena can hardly be overestimated. Since the discovery and promulgation of Coulomb's law, the era of the study of electromagnetism, which is of great importance in modern life, began.
Excerpt characterizing Pendant
“They made me a proposition about you,” he said, smiling unnaturally. “I think you guessed,” he continued, “that Prince Vasily came here and brought with him his pupil (for some reason Prince Nikolai Andreich called Anatoly his pupil) not for my beautiful eyes.” Yesterday they made a proposition about you. And since you know my rules, I treated you. – How should I understand you, mon pere? - said the princess, turning pale and blushing. - How to understand! – the father shouted angrily. “Prince Vasily finds you to his liking for his daughter-in-law and makes a proposal to you for his pupil. Here's how to understand it. How to understand?!... And I’m asking you. “I don’t know how you are, mon pere,” the princess said in a whisper. - I? I? what am I doing? Leave me aside. I'm not the one getting married. What do you? This is what it would be good to know. The princess saw that her father looked at this matter unkindly, but at that very moment the thought came to her that now or never the fate of her life would be decided. She lowered her eyes so as not to see the gaze, under the influence of which she felt that she could not think, but could only obey out of habit, and said: “I wish only one thing - to fulfill your will,” she said, “but if my desire it was necessary to express... She did not have time to finish. The prince interrupted her. “And wonderful,” he shouted. - He will take you with a dowry, and by the way, he will capture m lle Bourienne. She will be a wife, and you... The prince stopped. He noticed the impression these words made on his daughter. She lowered her head and was about to cry. “Well, well, just kidding, just kidding,” he said. “Remember one thing, princess: I adhere to the rules that a girl has every right to choose.” And I give you freedom. Remember one thing: the happiness of your life depends on your decision. There's nothing to say about me. - Yes, I don’t know... mon pere. - Nothing to say! They tell him, he doesn’t just marry you, whoever you want; and you are free to choose... Go to your room, think it over and in an hour come to me and say in front of him: yes or no. I know you will pray. Well, maybe pray. Just think better. Go. Yes or no, yes or no, yes or no! - he shouted even as the princess, as if in a fog, staggered out of the office. Her fate was decided and decided happily. But what my father said about m lle Bourienne - this hint was terrible. It’s not true, let’s face it, but it was still terrible, she couldn’t help but think about it. She walked straight ahead through the winter garden, seeing and hearing nothing, when suddenly the familiar whisper of M lle Bourienne woke her up. She raised her eyes and, two steps away, saw Anatole, who was hugging the Frenchwoman and whispering something to her. Anatole, with a terrible expression on his beautiful face, looked back at Princess Marya and did not release the waist of m lle Bourienne, who did not see her, at the first second. "Who is here? For what? Wait!" Anatole’s face seemed to speak. Princess Marya looked at them silently. She couldn't understand it. Finally, M lle Bourienne screamed and ran away, and Anatole bowed to Princess Marya with a cheerful smile, as if inviting her to laugh at this strange incident, and, shrugging his shoulders, walked through the door leading to his half. An hour later Tikhon came to call Princess Marya. He called her to the prince and added that Prince Vasily Sergeich was there. The princess, when Tikhon arrived, was sitting on the sofa in her room and holding the crying Mlla Bourienne in her arms. Princess Marya quietly stroked her head. The beautiful eyes of the princess, with all their former calm and radiance, looked with tender love and regret at the pretty face of m lle Bourienne.