Today, there are quite a lot of different battery-powered devices. And it’s even more annoying when, at the most inopportune moment, our device stops working, because the batteries are simply dead, and their charge is not enough for the normal functioning of the device.
Buying new batteries every time is quite expensive, but trying to make a homemade device for charging finger batteries with your own hands is quite worth it.
Many craftsmen note that it is preferable to charge such batteries (AA or AAA) using direct current, because this mode is most beneficial in terms of safety for the batteries themselves . In general, the transferred charge power from the network is about 1.2-1.6 times the capacity of the battery itself. For example, a nickel-cadmium battery with a capacity of 1A/h will be charged with a current of 1.6A/h. Moreover, the lower the given power, the better for the charging process.
Manufacturing process
In the modern world, there are quite a lot of household appliances equipped with a special timer that counts down a certain period, then signaling its end. When making your own device for charging AA batteries, you can also use this technology , which will notify you when the battery charging process is complete.
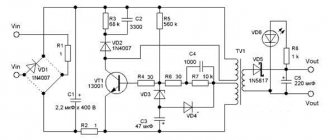
The charger for AA batteries is a device that generates direct current, charging up to 3 A/h. During production, the most common, even classic, scheme was used, which you see below. The basis, in this case, is the transistor VT1.
The voltage on this transistor is indicated by a red LED VD5, which acts as an indicator when the device is connected to the network. Resistor R1 sets a certain power of currents passing through this LED, as a result of which the voltage in it fluctuates. The value of the collector current is formed by the resistance from R2 to R5, which are included in VT2 - the so-called “emitter circuit”. At the same time, by changing the resistance values, you can control the degree of charging. R2 is constantly connected to VT1, setting a constant current with a minimum value of 70 mA. To increase the charging power, it is necessary to connect the remaining resistors, i.e. R3,R4 and R5.
It is worth noting that
the charger functions only when the batteries are connected .
After connecting the device to the network, a certain voltage appears on resistor R2, which is transmitted to transistor VT2. Then, the current flows further, as a result of which the VD7 LED begins to burn intensely.
How to recharge batteries at home
Many users of household appliances and electronic gadgets think about how to charge the battery so that it works again. Not all batteries can be charged, so you need to look at their labeling.
What batteries can be charged in the charger?
After working for the allotted time, the battery stops supplying electric current. This is due to the fact that the chemical processes occurring in it are irreversible.
But there is no need to throw away the battery; it can still serve if you put it in another device that does not require a full charge to operate, for example, a television remote control.
But there are galvanic cells that recharge many times. These are rechargeable batteries; manufacturers label them as rechargeable batteries. If we compare such cells with conventional batteries, their operating voltage is lower. The more recharge cycles they can withstand, the higher their cost.
To recharge them, you will need a charger. It is purchased separately and is equipped with an indicator that shows the battery charge level. It will take 8 to 12 hours to fully charge the battery.
Batteries that can be charged in a charger.
Recharging at home
To ensure that the battery lasts a long time, you need to pay attention to its capacity when purchasing. The larger it is, the longer the element will function. Manufacturers use the letters mAh to denote this indicator.
A rechargeable battery (AKB) is more expensive than conventional batteries due to the possibility of repeated charging. Simple batteries cannot be recharged, but craftsmen have come up with several ways to solve the problem.
If you need to charge batteries, do the following.
For emergency charging, use a charger designed for 4 batteries. Alkaline cells that need to be recharged are inserted from left to right. They are placed in the first three compartments.
The battery is placed in the fourth slot, located on the right. Charging time is 5-10 minutes. After this, the batteries are removed. They will work again, but it won't last long.
This method is considered relatively safe, but it cannot be called effective.
The chemical reactions inside batteries cannot be reversed. You can only slightly influence the electrolyte and extract a few percent of the power from it.
To do this you need to crush the battery. They take it with pliers or knock it on a hard object. But you need to do this carefully so that the case is not damaged. If there is strong compression or impact, the electrolyte will leak out and the battery will not function.
It is prohibited to heat the batteries, this will lead to an explosion. If you want to extend the life of the elements, you do not need to use them in the cold. At negative temperatures, charge is lost quickly.
Galvanic cells are discharged if they are not used for a long time, so you should find out about the shelf life when purchasing. Batteries will last less if old and new batteries are inserted into a household appliance at the same time or if different types of devices are combined.
Recharging batteries using a charger at home.
Using special devices
You can charge alkaline batteries using special devices. Battery Wizard is in demand among buyers. Consumers who bought it note that the cost of purchasing new galvanic cells has decreased.
The device can be of different shapes, for example round, rectangular or square. To recharge the battery, it is placed inside. After this, Battery Wizard is connected to a household network.
You need to keep an eye on the batteries being charged. When they become slightly warm, they are pulled out. They must not be allowed to overheat.
Danger of charging batteries
The batteries contain caustic alkali. When charging, when electric current passes through it, the battery may explode. This must be taken into account when charging in a room, garage or other enclosed space.
What can happen when charging batteries.
Another nuisance that may arise during the charging process is electrolyte leakage. If a small amount of the substance comes into contact with the device, it will be damaged.
When purchasing galvanic cells, you need to take into account that only alkaline (alkaline) cells can be recharged. It is impossible to extend the service life of salt products. Recharging them is considered dangerous. They may explode, causing electrolyte to splash into your eyes.
Is it possible to extend the service life
You can check the battery charge with a multimeter. If the battery is rechargeable, you need to use special devices for charging. They will independently calculate how long it takes to restore the charge. Usually at least 8-14 hours.
AA batteries can be easily recharged as follows:
- Take 2 wires and a galvanic cell with a full charge.
- Connect plus with plus, and minus with minus. After some time, the old battery will be powered by the new one.
An effective way is to use a power supply from a mobile phone, player or other equipment that is used to supply voltage from 1.5 to 3 V. The battery is connected to the existing terminals or the connector is cut off to create a plus and minus.
When connecting the power supply to the network, you must observe polarity. If you connect the minus to the plus, the battery will be discharged even more.
The battery must be charged until it reaches 50°C. After that, turn it off and wait for it to cool down. Then they are connected again, but kept on recharging for no more than 2 minutes. Turn it off again, and then put it in the freezer for 10 minutes. Take the battery out of the refrigerator and wait until it warms up naturally in the room. After this, it can be installed in devices.
An alternative way to charge alkaline batteries is to place them in hot water. You don’t need to hold it for a long time, 20 seconds is enough.
There is one more trick. Salted water is poured into the container and the battery is placed. Place the pan on the stove and boil the solution for several minutes. After this, the charged batteries are taken out, wrapped in electrical tape, and then installed in household appliances.
You can charge the battery by introducing chemically active substances into it. To do this, several holes are made in the lid, placing them along the graphite rod. The puncture depth should not exceed ¾ of the battery depth. The easiest way to work is with an awl; you can use it to measure the required length of the puncture in advance.
When the holes are ready, active liquid is poured into them. It could be vinegar. If it is not there, use hydrochloric acid at a concentration of 8-10%. After pouring, wait 2-3 minutes, during which time the reagents will be absorbed into the powder. The procedure is repeated 2-3 times.
Using reagents, you can restore the charge level to 80% of the factory one. To secure the result, the drilled holes are sealed with plasticine or sealant.
All of the above methods are labor-intensive, but with their help you can charge even flat tablets and little finger batteries. While in the city, it’s easier to buy a new battery, but when you’re on a hike, you can use these developments when an emergency arises.
Charging from USB port
You can make a charger for nickel-cadmium batteries based on a regular USB port . At the same time, they will be charged with a current of approximately 100 mA. The scheme, in this case, will be as follows:
At the moment, there are quite a lot of different chargers sold in stores, but their cost can be quite high. Considering that the main point of various homemade products is precisely to save money, self-assembly is even more advisable in this case.
This circuit can be modified by adding an additional circuit to charge a pair of AA batteries. Here's what we ended up with:
To make it more clear, here are the components that were used during the assembly process:
It is clear that we cannot do without basic tools, so before starting assembly you need to make sure that you have everything you need:
- soldering iron;
- solder;
- flux;
- tester;
- tweezers;
- various screwdrivers and knife.
Interesting material about making it yourself, we recommend viewing it
A tester is necessary to check the performance of our radio components. To do this, you need to compare their resistance, and then check it with the nominal value.
For assembly we will also need a case and a battery compartment. The latter can be taken from the children's Tetris simulator, and the body can be made from a regular plastic case (6.5cm/4.5cm/2cm).
We attach the battery compartment to the case using screws. The board from the Dandy console, which needs to be cut out, is perfect as a basis for the circuit. We remove all unnecessary components, leaving only the power socket. The next step is to solder all the parts based on our diagram.
The power cord for the device can be taken from a regular computer mouse cord with a USB input, as well as part of the power cord with a plug. When soldering, polarity must be strictly observed, i.e. solder plus to plus, etc. We connect the cord to USB, checking the voltage supplied to the plug. The tester should show 5V.
Choosing a charger
And finally, let’s look at which chargers are chosen by users of devices powered by rechargeable batteries. The table was compiled according to the degree of popularity of the latter.
Rating of chargers by popularity
| Rating | Appearance | Model | Manufacturer | Battery type | Battery size | Number of compartments (independent channels) | Additional functions | price, rub. | Where can I buy |
| 1 | Nitecore D4 | TM Nitecore | IMR/Li-ion, Ni-MH/Ni-Cd | AA (R6), AAA (R03), AAAA, C (R14), 26650, 22650, 18650, 10440, 14500, 16340, CR123A, 17670, 17500, 18490, 18350 | 4 | Automatic type detection, status display on LCD display, capacity detection, restoration, protection against polarity reversal, overcharge, overdischarge, powered by car cigarette lighter | 2 200 | Ya.Market | |
| 2 | Liitokala Lii-500 | Liitokala | Li-ion, Ni-MH | 18650, 18490, 18350, 17670, 17500, 17335, 16340 (RCR123), 14500, 10440, 26650, 22650, 26500, A, AA, AAA, SC | 4 | Automatic type detection, LCD status display, test function, recovery, reverse polarity protection, overcharge, overdischarge protection, USB port 5V/1A | 2 700 | Ya.Market | |
| 3 | Palo P10 | Palo | NI-MH, NI-CD | AA, AAA | 8 | LED charge indication, fixed charging current (200 or 180 mA), low cost, pair charging, pair auto-off at the end of charging | 900 | ||
| 4 |
How many batteries should I take?
To make a simple charger for a car battery, you need to calculate how many lithium batteries you need to take.
One barrel has a voltage of 3.7 Volts and weighs approximately 100 grams. The capacity differs and can vary within 1.505 Ah. Not enough for a car, but you can simply take more batteries to meet all power indicators.
The car requires a pulse charger consisting of three batteries. The total voltage should be 11-12 Volts. But it’s better to pay attention to capacity indicators. For car batteries it is approximately 60 Ah.
Three batteries provide 5 Ah. This means that the required voltage and current can be obtained using 38-40 of these batteries. They are quite enough to charge the car battery.
DIY fan: how to make a homemade powerful fan. Basic parameters and properties of fans (130 photos)- Why do you need a security alarm, what functions does it perform?
How to choose winter workwear and not make a mistake - recommendations from the pros
Do-it-yourself charger for AA batteries - a metalworker's guide
Today, there are quite a lot of different battery-powered devices. And it’s even more annoying when, at the most inopportune moment, our device stops working, because the batteries are simply dead, and their charge is not enough for the normal functioning of the device.
Buying new batteries every time is quite expensive, but trying to make a homemade device for charging finger batteries with your own hands is quite worth it.
Many craftsmen note that it is preferable to charge such batteries (AA or AAA) using direct current, because this mode is most beneficial in terms of safety for the batteries themselves .
In general, the transferred charge power from the network is about 1.2-1.6 times the capacity of the battery itself. For example, a nickel-cadmium battery with a capacity of 1A/h will be charged with a current of 1.6A/h.
Moreover, the lower the given power, the better for the charging process.
- 1 Manufacturing process
- 2 Charging from USB port
- 3 Conclusion
Features of chargers for AA batteries
Using ordinary batteries is unprofitable, since their service life is very limited. Therefore, it is more practical to use batteries.
Their advantage lies in repeated use, provided they are handled correctly. First of all, this is due to the conditions for their recharging.
Batteries, releasing accumulated energy to devices, periodically need to be charged. This is what battery chargers are for.
History of chargers
The discovery of galvanic electricity led to the creation of the first prototype of rechargeable batteries. In 1798, Italian physicist Alessandro Volta conducted an experiment involving placing copper and zinc plates connected in series in an acid solution.
He discovered that when current was passed through the plates after it was interrupted, a residual charge remained on them. Subsequently, Gotero, Marianini, and Becquerel became interested in these experiments.
But it was only in 1859 that Plante created the truly first battery..
His experiment was based on strips of lead with a piece of cloth sandwiched between them. He then rolled the strips and immersed them in acidified water.
Further development led to the fact that when the plates were coated with lead oxides, the formation of the active layer improved.
In 1896, the American company National Carbon Company (NCC) was the first in the world to begin producing batteries. Today it is known as Energizer. Early in 1901, scientist Thomas Edison patented a nickel-cadmium type of battery.
At the same time, Waldmar Jungner was developing a nickel-iron type called an alkaline battery. Alkaline batteries are used in transport and power plants.