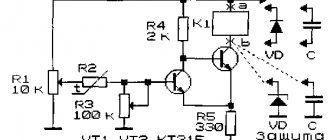
A directed flow of charged particles circulates through conductors made of different materials under different environmental conditions.
And the amount of charge is influenced by the resistance value of the conductors, which correlates with external parameters. These parameters also include air temperature.
Next, we will examine this issue in more detail.
Metals
How do metal materials react to temperature changes: heating or cooling? For consideration, the following experiment was simulated: a circuit was assembled from a wire, an ammeter, a wire, a battery and a heating burner.
To take the initial readings, the current strength in the assembled circuit is measured. Next, use a torch to heat the metal spiral. As the temperature of the wire increases, the resistance increases, and accordingly the current readings drop.
To experiment on the effect of temperature on the resistance of conductors, take:
- Spiral made of metal wire
- Power element
- Ammeter
In the video you can see the experiment described above.
There is such a parameter as the superconductivity of conductors. Under normal environmental conditions and a decrease in metal temperature, the resistance drops.
You can see this dependence of resistance on temperature using the example of mercury. At an extremely low temperature of the conductor, the resistance value rapidly drops, approaching zero. This is the phenomenon of superconductivity.
INFOFIZ
According to the value of electrical resistivity, semiconductors
occupy an intermediate place between conductors and dielectrics. Semiconductors include many chemical elements (germanium, silicon, selenium, tellurium, arsenic, etc.), a huge number of alloys and chemical compounds.
The qualitative difference between semiconductors and metals is manifested primarily in the dependence of resistivity on temperature. As the temperature decreases, the resistance of metals decreases. In semiconductors, on the contrary, the resistance increases with decreasing temperature and near absolute zero they practically become insulators.
Dependence of resistivity ρ of a pure semiconductor on absolute temperature T
.
Semiconductors
are substances whose resistivity decreases with increasing temperature.
Such a course of the dependence ρ( T
) shows that in semiconductors the concentration of free charge carriers does not remain constant, but increases with increasing temperature. The mechanism of electric current in semiconductors cannot be explained within the framework of the free electron gas model. An explanation of the phenomena observed in conductors is possible on the basis of the laws of quantum mechanics. Let us qualitatively consider the mechanism of electric current in semiconductors using germanium (Ge) as an example.
Germanium atoms have four weakly bound electrons in their outer shell. They are called valence electrons
.
In a crystal lattice, each atom is surrounded by its four nearest neighbors. The bond between atoms in a germanium crystal is covalent
, that is, it is carried out by pairs of valence electrons. Each valence electron belongs to two atoms.
The valence electrons in a germanium crystal are much more strongly bound to the atoms than in metals; Therefore, the concentration of conduction electrons at room temperature in semiconductors is many orders of magnitude lower than in metals. Near absolute zero temperature in a germanium crystal, all electrons are occupied in the formation of bonds. Such a crystal does not conduct electric current. As the temperature increases, some of the valence electrons may gain enough energy to break covalent bonds. Then free electrons
(conduction electrons). At the same time, vacancies are formed in places where bonds are broken, which are not occupied by electrons.
Vacancies that are not occupied by electrons are called
holes
.
The vacant place can be occupied by a valence electron from a neighboring pair, then the hole moves to a new place in the crystal. electron-hole pairs are formed per unit time
.
At the same time, the reverse process occurs - when a free electron meets a hole, the electronic bond between the germanium atoms is restored. This process is called recombination
.
Recombination is
the restoration of electronic bonds between atoms.
Electron-hole pairs can also be created when a semiconductor is illuminated due to the energy of electromagnetic radiation.
In the absence of an electric field, conduction electrons and holes participate in chaotic thermal motion.
If a semiconductor is placed in an electric field, then not only free electrons are involved in the ordered movement, but also holes, which behave like positively charged particles. Therefore, the current I
in a semiconductor it consists of electron
In
and hole
Ip
currents:
I
=
In
+
Ip
Electric current in semiconductors
is the directional movement of electrons to the positive pole, and holes to the negative.
The concentration of conduction electrons in a semiconductor is equal to the concentration of holes: nn
=
np
.
The electron-hole conductivity mechanism appears only in pure (that is, without impurities) semiconductors. It is called the intrinsic electrical conductivity
of semiconductors.
The intrinsic electrical conductivity
of semiconductors is called the electron-hole conduction mechanism, which manifests itself only in pure (that is, without impurities) semiconductors.
In the presence of impurities, the electrical conductivity of semiconductors changes greatly.
Impurity conductivity
is the conductivity of semiconductors in the presence of impurities.
A necessary condition for a sharp decrease in the resistivity of a semiconductor upon the introduction of impurities is the difference in the valence of the impurity atoms from the valence of the main atoms of the crystal.
There are two types of impurity conductivity - electronic
and
hole
conductivity.
- Electronic conductivity
occurs when an impurity with a higher valency is introduced into a semiconductor crystal.
For example, pentavalent arsenic atoms, As, are introduced into a germanium crystal with tetravalent atoms.
The figure shows a pentavalent arsenic atom found in a site of the germanium crystal lattice. The four valence electrons of the arsenic atom are included in the formation of covalent bonds with four neighboring germanium atoms. The fifth valence electron turned out to be extra; it easily breaks away from the arsenic atom and becomes free. An atom that has lost an electron becomes a positive ion located at a site in the crystal lattice.
Donor impurity
is an impurity of atoms with a valence exceeding the valence of the main atoms of the semiconductor crystal.
As a result of its introduction, a significant number of free electrons appear in the crystal. This leads to a sharp decrease in the resistivity of the semiconductor - thousands and even millions of times. The resistivity of a conductor with a high content of impurities may approach that of a metal conductor.
In a germanium crystal with an admixture of arsenic, there are electrons and holes responsible for the crystal’s own conductivity. But the main type of free charge carriers are electrons detached from arsenic atoms. In such a crystal nn
>>
n.p.
.
Conduction in which the main carriers of free charge are electrons is called
electronic conductivity.
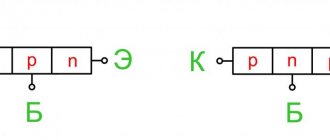
A semiconductor that exhibits electronic conductivity is called
an n-type semiconductor
.
- Hole conduction
occurs when an impurity with a lower valence is introduced into a semiconductor crystal.
For example, trivalent In atoms are introduced into a germanium crystal.
The figure shows an indium atom that has created covalent bonds with only three neighboring germanium atoms using its valence electrons. The indium atom does not have an electron to form a bond with the fourth germanium atom. This missing electron can be captured by the indium atom from the covalent bond of neighboring germanium atoms. In this case, the indium atom turns into a negative ion located at a site of the crystal lattice, and a vacancy is formed in the covalent bond of neighboring atoms.
Acceptor impurity
is an impurity of atoms with a valence lower than the valence of the main atoms of a semiconductor crystal capable of capturing electrons.
As a result of the introduction of an acceptor impurity, many covalent bonds are broken in the crystal and vacancies (holes) are formed. Electrons from neighboring covalent bonds can jump to these places, which leads to chaotic wandering of holes throughout the crystal.
The presence of an acceptor impurity sharply reduces the resistivity of the semiconductor due to the appearance of a large number of free holes. The concentration of holes in a semiconductor with an acceptor impurity significantly exceeds the concentration of electrons that arose due to the mechanism of the semiconductor’s own electrical conductivity: np
>>
nn
.
Conduction in which the main free charge carriers are holes is called
hole conductivity
.
A hole-conducting semiconductor is called
a p-type semiconductor
.
It should be emphasized that hole conductivity is actually caused by the movement of electrons through vacancies from one germanium atom to another, which carry out a covalent bond.
Dependence of electrical conductivity of semiconductors on temperature and illumination
- In semiconductors,
the mobility of electrons and holes decreases with increasing temperature, but this does not play a noticeable role, since when the semiconductor is heated, the kinetic
energy of the valence electrons increases and individual bonds break, which leads to an increase in the number of free electrons, i.e., an increase in electrical conductivity
.
- When a semiconductor is illuminated, additional carriers appear in it, which leads to an increase in its electrical conductivity.
This occurs as a result of light stripping electrons from the atom and simultaneously increasing the number of electrons and holes.
Read about what processes occur when p-n-type semiconductors come into contact and where semiconductors are used in the continuation of lecture 32 “Semiconductor diode.” Semiconductor devices»
Gases
Gaseous substances are not capable of transmitting electrical current. For its formation, charged particles are needed. Such carriers are called ions and are formed under the influence of environmental conditions.
We will analyze this situation in detail in the next experiment. We will need the same devices as for the previous one, only instead of wire there will be metal plates with small gaps between each other.
Under initial conditions, the measuring instrument indicates zero. After installing the burner between the conductors, you can observe an increase in current strength.
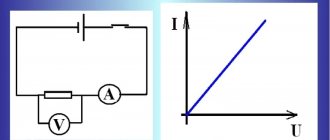
Above you can see an image of the current-voltage characteristics of a discharge in a gaseous environment. The graph clearly shows that after a rapid increase, the current indicators become stable, despite the change in voltage.
At the end there is a sharp jump, the result of which may be the destruction of the insulating layer.
Now let’s look at the practical side of the process of changing resistance as the temperature of the conductor increases. A simple example of application in everyday life is fluorescent lighting sources.
Two conductors, called the cathode and the anode, are placed in a flask containing an inert gas.
What happens when you turn it on? When receiving power, the conductors heat up and thermionic emission occurs. The inside of the vessel has a special phosphor coating, which provides the glow.
How do mercury and phosphor interact? When electrons and mercury vapor collide, infrared radiation is produced, which provides the glow. When voltage is applied to the cathode and anode, we obtain the phenomenon of gas conductivity.
How does the resistance of a metal conductor depend on temperature? What is the reason for this phenomenon?
It has been established that with increasing temperature the resistance of metal conductors increases, and with decreasing temperature it decreases. This increase or decrease in resistance for pure metal conductors is almost the same and averages 0.4% per 1°C. ... This explains an interesting phenomenon - the superconductivity of metals.
Interesting materials:
Why did Svyatoslav say I'm coming to you? Why do they say tailwind and seven feet under keel? Why do they say damn in Russia? Why do Latin Americans mostly speak Spanish and Portuguese? How many days after Easter can we say Christ is risen? How old is Dmitry Borisov, the host of the Let Them Talk program? How many megabytes are spent when talking on WhatsApp? How many times must we say Christ is risen? How much does a minute of conversation cost from Russia to Belarus? How much does a minute of conversation cost from Russia to Kazakhstan?
Liquids
Anions and cations are the key to current conductivity in a liquid due to the action of an electric field. Electrons in this case have little conductivity value. So, let's move on to the ratio of resistance to temperature of the liquid conductor.
Suitable for this experience:
- Electrolyte.
- Battery.
- A device for measuring current strength.
It is worth remembering this pattern when charging autonomous power sources, that is, batteries.