1.13. Electric current in semiconductors
In terms of electrical resistivity, semiconductors occupy an intermediate position between good conductors and dielectrics. Semiconductors include many chemical elements (germanium, silicon, selenium, tellurium, arsenic, etc.), a huge number of alloys and chemical compounds. Almost all inorganic substances in the world around us are semiconductors. The most common semiconductor in nature is silicon, which makes up about 30% of the earth's crust.
The qualitative difference between semiconductors and metals is manifested primarily in the dependence of resistivity on temperature. As the temperature decreases, the resistance of metals decreases (see Fig. 1.12.4). In semiconductors, on the contrary, the resistance increases with decreasing temperature and near absolute zero they practically become insulators (Fig. 1.13.1).
| Figure 1.13.1. Dependence of resistivity ρ of a pure semiconductor on absolute temperature T |
This behavior of the ρ(T) dependence shows that in semiconductors the concentration of free charge carriers does not remain constant, but increases with increasing temperature. The mechanism of electric current in semiconductors cannot be explained within the framework of the free electron gas model. Let us qualitatively consider this mechanism using the example of germanium (Ge). In a silicon (Si) crystal the mechanism is similar.
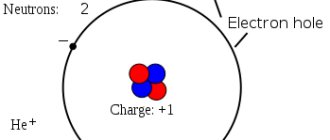
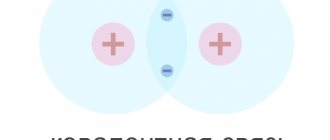
Germanium atoms have four weakly bound electrons in their outer shell. They are called valence electrons. In a crystal lattice, each atom is surrounded by its four nearest neighbors. The bond between atoms in a germanium crystal is covalent, that is, it is carried out by pairs of valence electrons. Each valence electron belongs to two atoms (Fig. 1.13.2). Valence electrons in a germanium crystal are much more strongly bound to atoms than in metals; Therefore, the concentration of conduction electrons at room temperature in semiconductors is many orders of magnitude lower than in metals. Near absolute zero temperature in a germanium crystal, all electrons are occupied in the formation of bonds. Such a crystal does not conduct electric current.
| Figure 1.13.2. Pair-electron bonds in a germanium crystal and the formation of an electron-hole pair |
As the temperature increases, some of the valence electrons may gain enough energy to break covalent bonds. Then free electrons (conduction electrons) will appear in the crystal. At the same time, vacancies are formed in places where bonds are broken, which are not occupied by electrons. These vacancies are called holes. The vacant place can be occupied by a valence electron from a neighboring pair, then the hole will move to a new place in the crystal. At a given semiconductor temperature, a certain number of electron-hole pairs are formed per unit time. At the same time, the reverse process occurs - when a free electron meets a hole, the electronic bond between the germanium atoms is restored. This process is called recombination. Electron-hole pairs can also be created when a semiconductor is illuminated due to the energy of electromagnetic radiation. In the absence of an electric field, conduction electrons and holes participate in chaotic thermal motion.
If a semiconductor is placed in an electric field, then not only free electrons are involved in the ordered movement, but also holes, which behave like positively charged particles. Therefore, the current I in a semiconductor consists of the electron In and hole currents Ip:
| I = In + Ip. |
The concentration of conduction electrons in a semiconductor is equal to the concentration of holes: nn = np. The electron-hole conductivity mechanism manifests itself only in pure (i.e., without impurities) semiconductors. It is called the intrinsic electrical conductivity of semiconductors.
In the presence of impurities, the electrical conductivity of semiconductors changes greatly. For example, adding phosphorus impurities in an amount of 0.001 atomic percent to a silicon crystal reduces the resistivity by more than five orders of magnitude. Such a strong influence of impurities can be explained on the basis of the above ideas about the structure of semiconductors.
A necessary condition for a sharp decrease in the resistivity of a semiconductor upon the introduction of impurities is the difference in the valence of the impurity atoms from the valence of the main atoms of the crystal.
The conductivity of semiconductors in the presence of impurities is called impurity conductivity. There are two types of impurity conductivity – electronic and hole.
Electronic conductivity occurs when pentavalent atoms (for example, arsenic, As) are introduced into a germanium crystal with tetravalent atoms.
| Figure 1.13.3. An arsenic atom in a germanium lattice. n-type semiconductor |
In Fig. Figure 1.13.3 shows a pentavalent arsenic atom found in a site of the germanium crystal lattice. The four valence electrons of the arsenic atom are included in the formation of covalent bonds with four neighboring germanium atoms. The fifth valence electron turned out to be redundant; it easily breaks away from the arsenic atom and becomes free. An atom that has lost an electron becomes a positive ion located at a site in the crystal lattice. An impurity of atoms with a valence exceeding the valence of the main atoms of a semiconductor crystal is called a donor impurity. As a result of its introduction, a significant number of free electrons appear in the crystal. This leads to a sharp decrease in the resistivity of the semiconductor - thousands and even millions of times. The resistivity of a conductor with a high content of impurities may approach that of a metal conductor.
In a germanium crystal with an admixture of arsenic, there are electrons and holes responsible for the crystal’s own conductivity. But the main type of free charge carriers are electrons detached from arsenic atoms. In such a crystal nn >> np. Such conductivity is called electronic conductivity, and a semiconductor that has electronic conductivity is called an n-type semiconductor.
| Figure 1.13.4. An indium atom in a germanium lattice. p-type semiconductor |
Hole conduction occurs when trivalent atoms (for example, indium atoms, In) are introduced into a germanium crystal. In Fig. Figure 1.13.4 shows an indium atom that, with the help of its valence electrons, has created covalent bonds with only three neighboring germanium atoms. The indium atom does not have an electron to form a bond with the fourth germanium atom. This missing electron can be captured by the indium atom from the covalent bond of neighboring germanium atoms. In this case, the indium atom turns into a negative ion located at a site of the crystal lattice, and a vacancy is formed in the covalent bond of neighboring atoms. An impurity of atoms capable of capturing electrons is called an acceptor impurity. As a result of the introduction of an acceptor impurity, many covalent bonds are broken in the crystal and vacancies (holes) are formed. Electrons from neighboring covalent bonds can jump to these places, which leads to chaotic wandering of holes throughout the crystal.
The presence of an acceptor impurity sharply reduces the resistivity of the semiconductor due to the appearance of a large number of free holes. The concentration of holes in a semiconductor with an acceptor impurity significantly exceeds the concentration of electrons that arose due to the mechanism of the semiconductor’s own electrical conductivity: np >> nn. This type of conductivity is called hole conductivity. An impurity semiconductor with hole conductivity is called a p-type semiconductor. The main free charge carriers in p-type semiconductors are holes.
It should be emphasized that hole conductivity is actually due to the relay movement of electrons through vacancies from one germanium atom to another, which carry out a covalent bond.
For n- and p-type semiconductors, Ohm's law is satisfied in certain ranges of current and voltage, provided that the concentrations of free carriers are constant.
Why does the electrolyte resistance decrease as the temperature increases?
Electrolyte resistance decreases
with
increasing temperature
, because when heated, the number of molecules that break down into ions (positive and negative) increases.
Interesting materials:
How to delete viewed pages on Instagram? How to delete viewed status in whatsapp? How to delete views on YouTube? How to remove Protectbrowser info? How to remove processes from task manager? How to remove Pubg lite? How to remove PUBG Mobile from PC? How to delete a post on Facebook from your phone? How to delete a post on Instagram from your computer? How to delete an Instagram post from your computer?