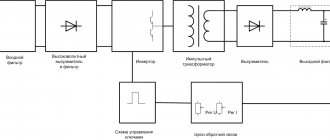
Electroplating is an electrochemical method of copying (obtaining exact copies of products). It is widely used in technology in the manufacture of matrices in printing, molds for pressing gramophone records, etc. This method is used to produce metal mesh, jewelry, copies of sculptures, engravings, and parts of complex configurations. The method is characterized by exceptionally high precision in reproducing the relief of the product.
Electroplating is an electrochemical process of coating one metal with another that is more mechanically and chemically stable, for example, steel parts are coated with chromium, nickel, copper parts with nickel, silver or other metals.
In principle, galvanoplasty is no different from electroplating. However, galvanostegic and galvanoplastic processes have their own characteristics and differ primarily in the methods of preparing the surface before deposition of metal on it.
In electroplating, the surface is prepared so that the coating adheres firmly to it. In electroforming, on the contrary, the coating must be easily separated. Therefore, in the latter case, much attention is paid to the application of conductive layers (in the case of coating non-conductors) and separating layers (if the copy is made from metal).
Further, while many metals and alloys are used for electroplating (silver, zinc, tin, nickel, copper, chromium and their alloys), in electroplating only deposits of copper, nickel and silver and much less often other metals are used. Due to the fact that galvanoplastic deposits differ from galvanostegic deposits by being much thicker, the compositions of electrolytes and modes used in electroplating also differ somewhat from those adopted in galvanostegy.
In galvanoplasty, metal is usually built up not onto metal, but onto a thin conductive layer deposited on the surface of a non-conductor, or onto a separating, poorly conductive layer deposited on the metal. Therefore, an additional operation of “tightening” with metal is introduced into the technological process compared to electroplating - primary buildup metal onto the conductive layer until it is completely covered. The compositions of electrolytes for puff baths and the operating mode are somewhat different from conventional ones.
Equipment and homemade devices
Equipment for electroplating is no different from equipment used for electroforming. Any glass can be used as a galvanic bath of such a size that the object to be coated with metal can be placed freely in it and not be too close to the anode plates.
Rice. 1. Galvanic bath in a quadrangular jar.
It is most convenient to use quadrangular glass jars (Fig. 1).
Transverse crossbars are made from thick copper wire or tubes, two of which (a) are used for hanging nickel or copper plates - anodes, and the third (b) - for nickel-plated or copper-plated objects.
Rice. 2. Galvanic bath in a round jar.
In a round jar, the anode plate has to be bent into a cylinder (c) (Fig. 2).
The objects to be coated are suspended on copper wires. There should be two anode plates. It is important that the objects to be coated face the anodes with their largest areas and are approximately in parallel planes with them.
The crossbars from which the anodes and coated objects are suspended must be equipped with terminals for convenience and reliability of connection (see Fig. 3). The wires that attach the anode to the crossbar must be above the level of the Electrolyte, especially if they are made of another metal.
The anode plates are connected in parallel to each other and must be connected to the “plus” terminal of the battery or rectifier current source).
Anodes must be thoroughly cleaned of oxides, dirt and degrease, just like objects intended to be coated with metal.
An important condition for successful nickel and copper plating is cleanliness. If slight turbidity appears in the electrolyte or a precipitate has formed, the electrolyte must be filtered.
In Fig. Figure 3 shows the connection diagram for the galvanic bath. As a source, you can use a car battery or a rectifier (voltage 6-12 V), powered by an alternating current network with a voltage of 127-220 V. A voltmeter and an ammeter must be connected to the circuit. If the surface of the object to be coated is less than 2 dm^2, you can use a 500 mA milliammeter.
The resistance of the rheostat should be about 8-10 Ohms so that the current can be changed within fractions of an ampere.
When assembling the electrical circuit of the bathtub, it is very important not to confuse the poles of the battery or rectifier, since the anode plates must be connected to the positive pole, and the part (object) to the negative. If turned on incorrectly, the metal of the part or object will “dissolve”, which will lead to damage to the electrolyte.
An even, dense coating of an object with nickel or copper depends on the magnitude of the electric current, which does not exceed a known limit and depends on the surface area of the object.
For example, if the standard current density is 0.5 A per 1 dm^2 and the object has a total surface of about 0.5 dm^2, then the current should not exceed 0.5 X 0.5 = 0.25 A. At higher currents nickel or copper will be deposited as a dark, weak layer that comes off easily. If the object has pointed parts, the current density should be reduced by 2-3 times.
Objects are immersed in a bath under voltage. To do this, they are first suspended on bare copper conductors with a diameter of 0.8-1 mm to a crossbar (copper tube), connected to a source of electric current (the rheostat is turned on to full resistance) and lowered into a bath of electrolyte. Then, by reducing the resistance of the rheostat, the current is brought to normal.
Rice. 3. Scheme of connecting a galvanic bath to an electrical circuit.
During galvanization, the part or object is removed from the bath for a short time two or three times and inspected. If the metal is deposited unevenly, change the position of the object, turning it towards the anode with the side on which the metal layer is thinner.
With the correct nickel plating process, the nickel is deposited in a matte, even, silvery layer throughout. The appearance of dark spots indicates poor degreasing. A thin layer of metal is deposited on a part or object in 20-30 minutes, a thick layer in several hours.
An object taken out of the bath, no matter how well it has been previously polished, has a matte surface. To add shine, it is polished with the finest chalk (tooth powder) using a cloth. You can also polish with crocus, but be very careful not to damage the nickel layer.
Note. Aluminum is widely used in amateur designs. Anodizing can be performed with alternating current 12-24 V. The part (sheet) is polished to a mirror shine, wiped with acetone and chemically degreased in a solution of caustic soda 50 g/l. Degreasing time is 3-5 minutes, solution temperature is 50° C.
AC anodizing is as follows. If a part (sheet) is anodized, then it is the first electrode, and the second can be a processed aluminum billet or sheet.
The contacts of the current leads must be aluminum. The electrolyte is a 20% sulfuric acid solution.
Anodizing conditions are as follows.
- For aluminum and clad duralumin, the current density is 1.5-2 A/dm^2 at a voltage of 12 V. Anodizing time is 25-30 minutes, electrolyte temperature is not higher than 25 ° C.
- For unclad duralumin, the current density is 2-3 A/dm^2 at a voltage of 12-20 V. Anodizing time is 20-25 minutes, electrolyte temperature is about 25 ° C.
Manufacturing process
We take approximately a 20-centimeter piece of multi-core cable and remove the wire from it. We protect the insulation on both sides of the wire, bend one end of it at an angle of 90 degrees and glue it to the plastic part with instant glue. Moreover, BF glue will not work, since bronze paint will dissolve it.
When the items are dry, we degrease them using household chemicals (for example, washing powder). Next, rinse the product in running water or treat it with acetone.
The parts are firmly fixed to the wire. Now they can be dipped one at a time into pre-prepared bronze paint or this material can be applied with a brush. The entire surface must be evenly painted. It is recommended to use insulated wire from the cable, otherwise copper will fall on the bare wire, which will lead to additional consumption of the anode.
After drying the surface for an hour, twist the dried ends of the wires together. The parts must not touch each other. Next, we connect the products to the positive contact and immerse them in the bath. A few seconds after immersion, the copper plating process, visible to the naked eye, will begin.
The thickness of the copper coating may vary depending on the circumstances, but for small items it will be approximately 0.05 millimeters. The parts are in the bath for 15 hours. The current is adjusted by moving the contact along the nichrome rheostat within 0.8-1.0 Amperes. After copper plating, we increase the current to 2 Amperes. When the curing period of the parts has expired, we wash the items in running water, dry them, and cut off the wire. We clean the wire and prepare it for the next procedure.
Metallization is complete. Next, take sulfur ointment (can be purchased at a pharmacy), apply it to the surface and carry the part over the fire of a gas stove. In this case, the copper will immediately darken.
The next stage is polishing. For this, a motor equipped with a metal round brush is useful. This job requires a certain skill. The result should be a surface that looks like blackened bronze with some shiny areas. If you cannot immediately achieve the desired result, apply sulfur ointment again, heat the product over the fire and polish it.
For those who doubt the effectiveness of the procedure described above, we suggest doing a test. To do this, you will need a container for electrolyte, where you need to put a little copper. Paint one part with a spray bottle in 2-3 layers in bronze color. Next you need to connect to the battery without using a rheostat. The adapter from the player will also work.
Electrolytic electroforming
First of all, an imprint is taken from the object or product being copied, that is, a mold is made from fusible metal, wax, plasticine or plaster. The object to be copied, rubbed with soap, is placed in a cardboard box and filled with Wood's low-melting alloy or other low-melting alloys.
After casting, the object is removed and the resulting mold is degreased and subjected to copper plating in an electrolytic bath. In order to-
To prevent metal from being deposited on those sides of the mold where there is no imprint, they are coated with molten wax or paraffin using a brush. After copper plating, the low-melting metal is melted in boiling water to form a matrix. The matrix is filled with plaster or lead, and the copy is ready.
The following wax composition is used to make molds:
- Wax - 20th century. h.
- Paraffin - 3rd century. h.
- Graphite - 1st century. h.
If the mold is made of dielectric (wax, plasticine, paraffin, gypsum), its surface is covered with an electrically conductive layer. The conductive layer can be applied by reducing certain metals (silver, copper, nickel) or mechanically - by rubbing flake graphite into the surface of the mold with a soft hair brush.
Graphite is thoroughly ground in a porcelain mortar, sifted through a sieve or gauze and applied to the surface of the product with a soft brush or cotton swab. Graphite sticks better to plasticine. Forms made of plaster, wood, glass, plastic and papier-mâché are coated with a solution of wax in gasoline.
Graphite powder is applied to the surface that has not had time to dry, and excess, non-adherent graphite is blown off.
The electroplated coating is easily separated from the graphite-coated mold. If the mold is made of metal, then on its surface it is necessary to create an electrically conductive film of oxide, sulfide or other insoluble salt, for example, on silver - silver chloride, on lead - lead sulfite, so that the mold is well separated from the coating.
Copper, silver and lead surfaces are treated with a 1% solution of sodium sulfide, as a result of which insoluble sulfides are formed on them.
Deposition of metal on the surface of the mold. The prepared form is immersed in a bath, the circuit of which is energized so that the separating film does not dissolve. First, a “tightening” (coating) of a conductive layer of copper is carried out at a low current density in a solution of the following composition:
- Copper sulfate (copper sulfate) - 150-200 g,
- Sulfuric acid - 7-15 g,
- Ethyl alcohol - 30-50 ml,
- Water - 1000 ml.
The operating temperature of the electrolyte is 18-25° C, the current density is 1-2 A/dm^2. Alcohol is necessary to increase surface wettability. After the entire surface is “covered” with a layer of copper, the mold is transferred to an electrolyte intended for electroplating.
For galvanoplastic work (copper plating), the following composition is recommended:
- Copper sulfate (copper sulfate) - 340 v. h.
- Sulfuric acid - 2 c. h.
- Water - 1000 v, h.
Electrolyte temperature 25-28° C. Current density 5-8 A/dm2.
THE INVENTION OF GALVANOSTEGY
It should be especially noted that Jacobi did not go unnoticed such a property of the galvanoplastic copper deposit as tight fusion with the electrode. He immediately realized that electroplating could be used to cover the surfaces of some metals with layers of other metals, i.e. electroplating. Already in 1839, Jacobi produced samples coated with a galvanic layer of metal, not only with a layer of copper, but also with gold, silver and platinum.
Through the work of its inventor, electroplating has grown into electroplating, which includes electrolytically reproducing the shape of objects, followed by separating the layer with the imprint (galvanoplasty) and coating one metal with a thin layer of another (electroplating).
For the sake of historical justice, it should be said that in 1842, i.e. two years after the publication of the first manual on Jacobi galvanoplasty, the University Printing House in Moscow published the first manual on electroplating, written by Alexei Fedorovich Grekov, “Theoretical and a practical guide to gilding, silvering, platinizing, tinning." In the same year, St. Petersburg dentist Briand proposed the first successful iron sulfide electrolyte for gilding. His method was devoted to the “Report on galvanic gilding”, made by Jacobi at a meeting of the physics and mathematics department of the Academy of Sciences on September 23, 1842. This method was later improved by A.F. Grekov and P.R. Bagration. In 1844, Prince V.F. Odoevsky, a writer, critic and popularizer of scientific knowledge, wrote the book “Galvanism in Technical Application.”
Rice. 4 Interior sculpture of St. Isaac's Cathedral.
Metallization of non-metallic objects, methods and tips
To make metal plant leaves, fresh leaves are imprinted on a wax composition as follows. The wax composition is poured into a mold made of thick paper and allowed to cool almost until completely hardened, but in such a way that its surface is elastic. Then the leaves are placed on the surface of the wax and pressed against the glass.
When the glass and leaves are removed, a clear imprint of the leaves remains on the wax composition.
After the wax has completely hardened, the form with the imprint is carefully graphitized with a soft brush. Having installed the conductors on the form, hang the load and lower it into the galvanic bath.
To coat insects (butterflies, beetles, etc.) with metal, they are prepared in the appropriate way: the insects are kept in a 1.5% solution of sublimate, dried, and coated with varnish or a thin layer of wax. Then the surface needs to be made conductive; to do this, it is lubricated with a brush with a liquid slurry of graphite diluted in alcohol or vodka. After drying, excess graphite is removed.
After this, the object is suspended on several thin copper wires with a diameter of 0.1-0.2 mm, twisting or tying them repeatedly crosswise (Fig. 1), and placed in a galvano-plastic bath. To eliminate buoyancy in butterfly electrolyte
ku, bug, etc. are attached with paraffin to glass or a piece of plastic. The metal begins to be deposited first of all near the copper wires, spreading very slowly to the rest of the surface.
Therefore, at the beginning of the process, the current should be several times less than normal, but when the entire surface is “covered” with metal, it is brought to normal. The duration of the process is several hours. The thickness of the coating can vary from 0.1 to 2 mm.
Rice. 1. Hanging a beetle for copper plating (a). View of a beetle covered with metal (b).
Using the electroplating method, lace can be metalized for decorative and artistic decoration of various objects.
The lace is stretched on a frame and impregnated with paraffin. They are then ironed between sheets of paper to remove excess paraffin. Next, an electrically conductive layer of fine graphite is applied, the excess of which is carefully blown off the lace.
Having laid the conductors along the edge of the lace, they are attached to a plastic frame or a frame made of thick wire with vinyl chloride insulation, along with which the lace is immersed in an electrolyte.
Lace coated with copper is treated with a brass brush. They are soldered with tin-lead solder. Electroplating finishing of metallized lace involves applying a decorative layer of silver or gold or oxidation.
Application of technology
Electroplating is often used in relation to various elegant objects (jewelry, orders and medals, coins, shells, flower pots, sculptures, portraits, etc.). Copper is most often used in electroforming. However, other metals can also be used, including nickel, chromium, steel, and silver.
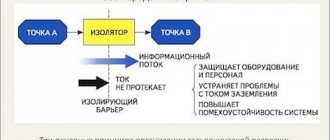
If all technological requirements are met, it is possible to distinguish a copied object from an original one only by the barrier layer or by removing the original. Moreover, it is quite possible to do all the work yourself at home.
Note! The coating of the copied product must be electrically conductive. If the material lacks this property, bronze or graphite is applied to it.
Galvanic coatings GOST
Galvanization is suitable for solving various problems; what it is from the point of view of professionals can be clarified in specialized standards. The necessary information is given in official standards.
Table of thematic GOSTs
| Document Number | Subjects, features |
| 9.309.-86 | Creation of uniform coatings at an average current density of no more than 5A per dm2. |
| 9.308-85 | Test technologies |
| 12.3.008-75 | Safety regulations |
| 9.005-72 | Acceptable combinations of metals that do not form a galvanic cell |
| 9.313-89 | Creation of coatings on products made of polymer materials |
| 9.908-85 | Determination of corrosion resistance for selection of galvanic isolation unit |
| 12.1.007-75 | Classification of harmful substances |
| ISO 4042 | Creation of electroplating coatings on fasteners |
| 2789-73 | Surface roughness |
Safety precautions during work
Be sure to check the compliance of the power supply network with high power consumption. If necessary, use a separate line, which is connected in the electrical panel to a separate circuit breaker. The DC source is grounded. Only serviceable equipment is used.
To carry out work safely, it is better to use a garage, other technical room, or an outdoor area. Additionally, standard personal protective equipment is used:
- latex gloves;
- respirators, gauze bandages;
- transparent masks, glasses;
- clothes with long sleeves.
Galvanic pair of electrodes
Galvanic cells are conductors made of different materials. The second prerequisite for this term is the connection of the circuit to ensure electrical contact and the generation of electromotive force between the contacts. Immersing such parts in a solution with clearly expressed alkaline (acidic) characteristics activates corrosion. To prevent rapid destruction, in addition to the copper-aluminum pair, the following combinations are not recommended:
- titanium-aluminium;
- tin-silver;
- lead-platinum;
- nickel-magnesium alloy, etc.
Purposes of Metal Electroplating
Capacitor energy
What is electroplating for household use? Theoretically, it is not too difficult to find a specialized enterprise, conclude an agreement, and receive a finished product with official guarantees. However, the practical implementation of such ideas is associated with various difficulties:
- payment for services and loss of time;
- lack of good specialists or relevant industries nearby;
- the reluctance of performers to reconfigure existing equipment to perform a relatively small amount of work.
Only you can create a unique electroplating with special characteristics yourself. Technology opens up vast opportunities for individual creativity. As will become clear after studying the data presented in the publication, the technology can be reproduced with high quality without excessive costs.
Galvanic coating provides an impeccable appearance for products with complex shapes
The photograph clearly demonstrates the excellent quality of processing of the smallest details and hard-to-reach areas. In addition to improving aesthetic parameters, metal electroplating helps create a layer of low electrical resistance on the insulator.
Stainless steel is expensive. Instead, the resistance of products at high humidity is increased with the help of copper plating. The technology is suitable for the production of spectacular jewelry, decorative and functional furniture elements. With its help, miniature parts are strengthened and chemical neutrality is ensured.
In cosmetology, low-intensity galvanic discharge is used to improve the functional state of the skin and remove individual defects.